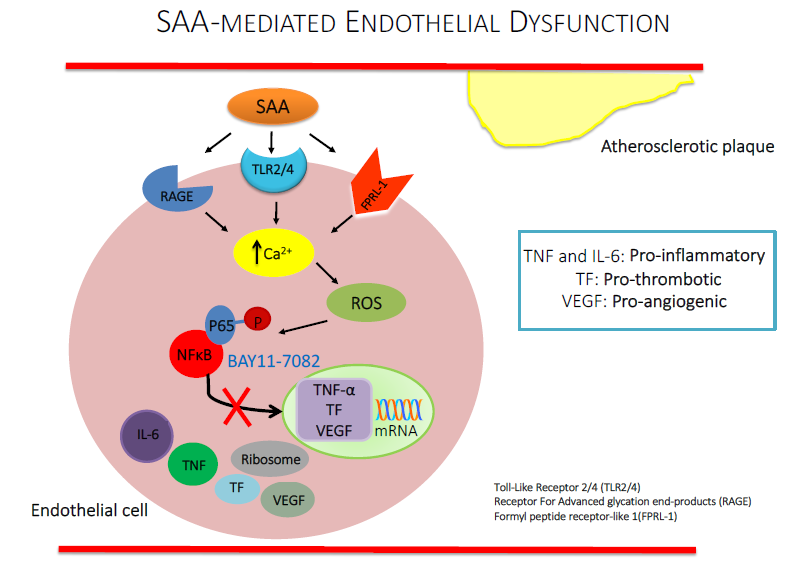
The acute phase protein serum amyloid A (SAA) is associated with endothelial dysfunction and early-stage atherogenesis. Stimulation of vascular cells with SAA increases gene expression of pro-inflammation cytokines and tissue factor (TF). Activation of the transcription factor, nuclear factor kappa-B (NFkB), may be central to SAA-mediated endothelial cell inflammation, dysfunction and pro-thrombotic responses, while targeting NFkB with a pharmacologic inhibitor, BAY11-7082, may mitigate SAA activity. Human carotid artery endothelial cells (HCtAEC) were pre-incubated (1.5 h) with 10 µM BAY11-7082 or vehicle (control) followed by SAA (10 μg/mL; 4.5 h). Under these conditions gene expression for TF and TNF increased in SAA-treated HCtAEC and pre-treatment with BAY11-7082 significantly (TNF) and marginally (TF) reduced mRNA expression. Intracellular TNF and IL-6 protein also increased in HCtAEC supplemented with SAA and this expression was inhibited by BAY11-7082. Supplemented BAY11-7082 also significantly decreased SAA-mediated leukocyte adhesion to apolipoprotein E-deficient mouse aorta in ex vivo vascular flow studies. In vascular function studies, isolated aortic rings pre-treated with BAY11-7082 prior to incubation with SAA showed improved endothelium-dependent vasorelaxation and increased vascular cGMP content. Together these data suggest that inhibition of NFkB activation may protect endothelial function by inhibiting the pro-inflammatory and pro-thrombotic activities of SAA.