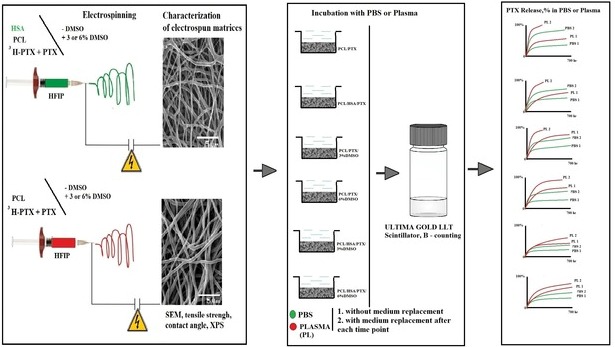
Paclitaxel is a natural, highly lipophilic anti proliferative drug widely used in medicine. We have studied the release of tritium-labeled paclitaxel (3H-PTX) from matrices destined for the coating of vascular stents and produced by the electrospinning method from the solutions of polycaprolactone (PCL) with paclitaxel (PTX) in hexafluoisoropropanol (HFIP) and/or solutions of PCL with PTX and human serum albumin (HSA) in HFIP or HIFP-dimethyl sulphoxide (DMSO) blend. The release of PTX has been shown to depend on the solvent and the composition of electrospinning solution, as well as the composition of the surrounding medium, particularly the concentration of free PTX and PTX-binding biomolecules present in human serum. It was shown that 3D matrices can completely release PTX without weight loss. Two-phase PTX release from optimized 3D matrices was obtained: ~27% of PTX was released in the first day, another 8% were released over the next 26 days. Wherein ~2.8%, ~2.3%, and ~0.25% of PTX was released on day 3, 9, and 27, respectively. Considering PTX toxicity, the rate of its diffusion through the arterial wall, and the data obtained the minimum cytostatic dose of the drug in the arterial wall will be maintained for at least three months.