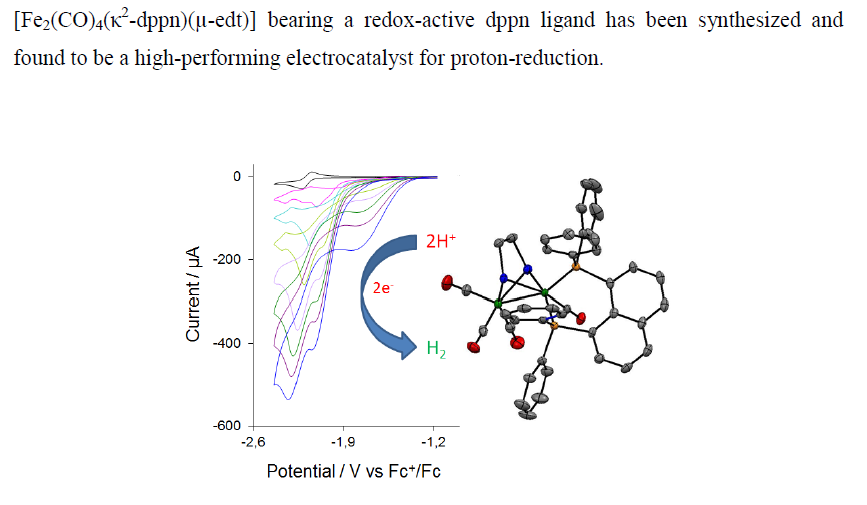
Addition of the bulky redox-active diphosphine 1,8-bis(diphenylphosphino)naphthalene (dppn) to [Fe2(CO)6(-edt)] (1) (edt = 1,2-ethanedithiolate) affords [Fe2(CO)4(2-dppn)(-edt)] (3) as the major product, together with small amounts of a P-C bond cleavage product [Fe2(CO)5{1-PPh2(1-C10H7)}(-edt)] (2). The redox properties of 3 have been examined by cyclic voltammetry and it has been tested as a proton-reduction catalyst. It undergoes a reversible reduction at E1/2 = –2.18 V and exhibits two overlapping reversible oxidations at E1/2 = –0.08 V and E1/2 = 0.04 V. DFT calculations show that while the HOMO is metal-centred (Fe-Fe -bonding), the LUMO is primarily ligand-based but also contains an antibonding Fe-Fe contribution, highlighting the redox-active nature of the diphosphine. It is readily protonated upon addition of strong acids to afford two isomeric hydride complexes and catalyzes the electrochemical reduction of protons at Ep = –2.00 V in the presence of CF3CO2H. The catalytic current indicates that it is one of the most efficient diiron electrocatalysts for the reduction of protons, albeit operating at quite negative potential.