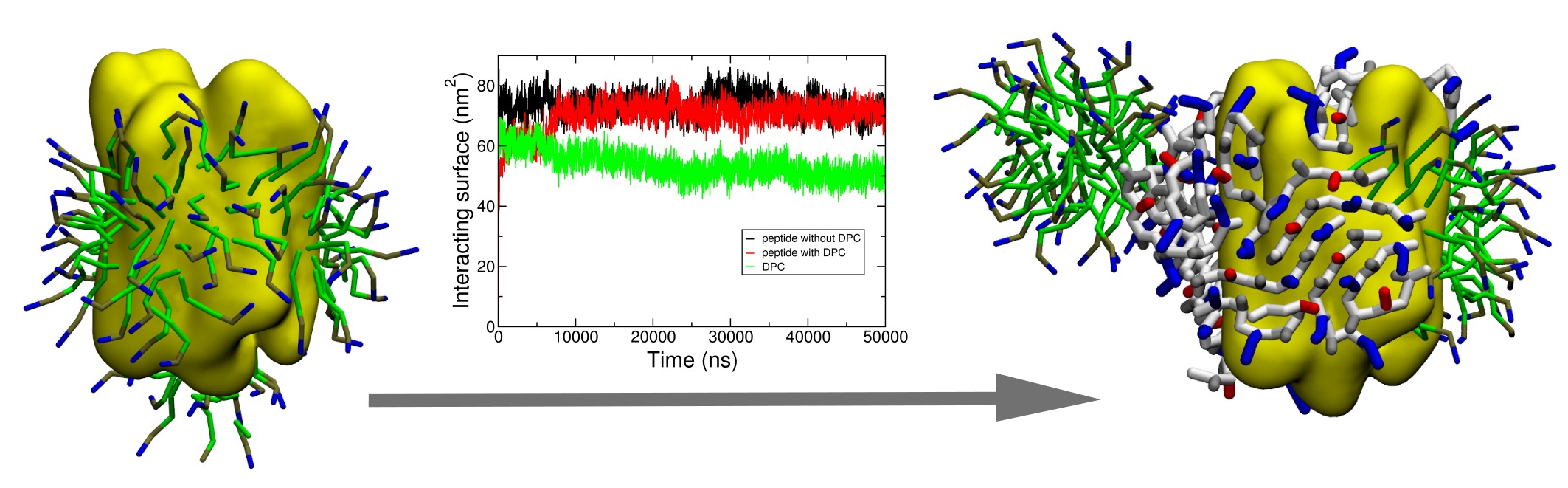
By manipulating the various physico-chemical properties of amino acids, design of peptides with specific self-assembling properties has been emerging since more than a decade. In this context, short peptides possessing detergent properties (so-called “peptergents”) have been developed to self-assemble into well-ordered nanostructures that can stabilize membrane proteins for crystallization. In this study, the peptide with “peptergency” properties, called ADA8 extensively described by Zhang et al., is studied by molecular dynamics for its self-assembling properties in different conditions. In water, it spontaneously forms beta sheets with a β barrel-like structure. We next simulated the interaction of this peptide with a membrane protein, the bacteriorhodopsin, in the presence or absence of a micelle of dodecylphosphocholine. According to the literature, the peptergent ADA8 is thought to generate a belt of β structures around the hydrophobic helical domain that could help stabilize purified membrane proteins. Molecular dynamics is here used to challenge this view and to provide further molecular details for the replacement of detergent molecules around the protein. To our best knowledge, this is the first molecular mechanism proposed for ''peptergency''. In addition, our calculation approach should serve as a predicting tool for the design of beta peptergent with diverse amphipathic properties.