1. Introduction
The global prevalence of chronic kidney disease (CKD) has increased substantially over the past decades, with the number of cases increasing by 104.09% in 2019 compared to 1990 [
1]. Moreover, CKD is now widely recognized as one of the leading causes of death worldwide, and it is projected that by 2040, it will become the fifth leading cause of mortality worldwide [
2]. Regarding the end-stage renal disease (ESRD) population, sudden cardiac death appears to be the leading cause of death, accounting for up to 50% of cases, while cardiac causes are at about 8.3% [
3].
Galectin-3 has been studied as a potential biomarker for predicting cardiovascular (CV) risk and mortality in hemodialysis (HD) patients. Galectin-3 is a β-galactoside-binding protein that is expressed in various tissues. It is located intracellularly or secreted into the extracellular space, and it plays a role in cell proliferation, differentiation, apoptosis, fibrosis and inflammation [
4]. Galectin-3 has been widely studied in cardiovascular disease, chronic kidney disease, but also in rheumatological conditions, such as rheumatoid arthritis, gastrointestinal diseases and idiopathic pulmonary fibrosis [
5]. A meta-analysis published in 2019 regarding the prognostic impact of galectin-3 in CKD patients showed that in CKD patients every 1% increase in galectin-3 increased all-cause mortality by 37.9%. However, this association was not consistent among HD patients [
6]. Most importantly, the impact of galectin-3 was not evaluated long-term in a population with ESRD on hemodialysis.
In a previous study, our research group combined NT-proBNP and gal-3 to develop a biomarker-model for better predicting CV outcomes in stable asymptomatic HD patients during a median follow-up of 36 months. Results suggested that the combination of NT-proBNP and gal-3 (one biomarker for fibrosis and one for myocardial stretch) correlated with major cardiovascular outcomes in stable HD patients [
7].
The current study aimed to reassess on a very long-term the same cohort of patients to evaluate all-cause mortality, major cardiovascular events (MACE) and an exploratory outcome: all-cause mortality stratified by pulse wave velocity (PVW) values.
2. Materials and Methods
This is a long-term (at least 10 years) observational study designed to assess all-cause mortality and MACE in a cohort of patients with ESRD on intermittent hemodialysis. The cohort included 173 clinically stable, asymptomatic patients receiving standard thrice-weekly HD treatment. Patients were included if they had been on HD for at least 3 months and were recruited from two HD units in Iasi, Romania. Exclusion criteria were: known ischemic heart disease (diagnosed by stress test or angiography), prevalent heart failure, active malignancy and acute infections. All participants in the original study provided written informed consent, and the study was approved by the local ethics committee of “Dr. C.I. Parhon” Hospital. The current study complies with the principles of the Declaration of Helsinki.
Follow-up and data collection
We conducted a long-term observational study (through October 1st, 2025) to evaluate survival and cardiovascular outcomes in the initial cohort. Vital status (alive or deceased) was verified through dialysis center records or by ascertaining the national electronic health database. For deceased patients, the presumed cause of death was determined from available clinical documentation, including hospital discharge summaries and dialysis unit reports. When cause of death was not identified or when the cause of death was sudden death (as reported by dialysis center records), those causes were excluded from the cause-specific cardiovascular mortality analysis but retained in the all-cause mortality dataset. Cardiovascular events (stroke, acute myocardial infarction, coronary revascularization procedures) were identified through electronic records from “Dr. C.I. Parhon” Hospital and corresponding dialysis centers. Regarding year of death, 3 records were missing.
The classification of patients into four subgroups according to baseline Gal3 and NT-proBNP levels —Group 1: low Gal3, low NT-proBNP; Group 2: high Gal3, low NT-proBNP; Group 3: low Gal3, high NT-proBNP; Group 4: high Gal3, high NT-proBNP was retained from the original study. The cut-off values for Gal3 and NT-pro-BNP (28.1 ng/mL and 4234pg/mL pg/mL, respectively) were based on their respective median concentrations in the baseline cohort.
Outcomes
The primary outcome was all-cause mortality during follow-up. The secondary outcomes included: three-point MACE, defined as non-fatal stroke, non-fatal acute myocardial infarction and cardiovascular death. This definition was chosen to ensure the accuracy and reliability of the data, as detailed, verified information regarding heart failure hospitalization, arrhythmias, or unstable angina was not available for the entire cohort. We aimed to minimize misclassification bias and improve the robustness of the cardiovascular outcome assessment.
An exploratory analysis was conducted to assess the association between baseline PVW and long-term all-cause mortality, with the cut-off pf PVW being the median value of the included cohort (9.85m/s).
Statistical analysis
All statistical analyses were performed using JASP (version 0.95.4). Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR), as appropriate. Categorical variables were summarized as absolute numbers and percentages. The normality of continuous variables was assessed using the Shapiro-Wilk test and visual inspection of histograms. Baseline differences across the four groups were assessed using one-way ANOVA for normally distributed continuous variables and Kruskal–Walli’s test for non-normally distributed continuous variables. Categorical variables were compared using χ² or Fisher’s exact test, as appropriate. A two-tailed p-value <0.05 was considered statistically significant.
All-cause mortality was analyzed using the Kaplan-Meier method, with comparisons between the four defined groups using the log-rank test. Cox proportional hazard regression was used to identify predictors for all-cause mortality. Covariates included age, sex, diabetes, hypertension, peripheral artery disease, dialysis vintage, pulse wave-velocity and the four defined groups. Results were reported as hazard ratios (HR) with 95% confidence intervals (CI). The proportional hazards assumption was verified using scaled Schoenfeld residuals and model-calibration was assessed through visual inspection of log-minus-log plots. MACE was analyzed as a binary outcome using logistic regression. The primary model included the same covariates in the Cox model. Model was assessed using the Hosmer-Lemeshow test and Nagelkerke’s R². A sensitivity analysis was performed using a parsimonious model (including only age, sex, diabetes and groups). The stability of estimates was evaluated by comparing odds ratios (OR) and confidence intervals (CI) between models. PVW was treated as a continuous variable (per 1m/s increase) and as a categorical variable dichotomized at 9.85m/s (the median value of the cohort). Sensitivity analyses were conducted to ensure the model's robustness.
3. Results
3.1. Baseline Characteristics
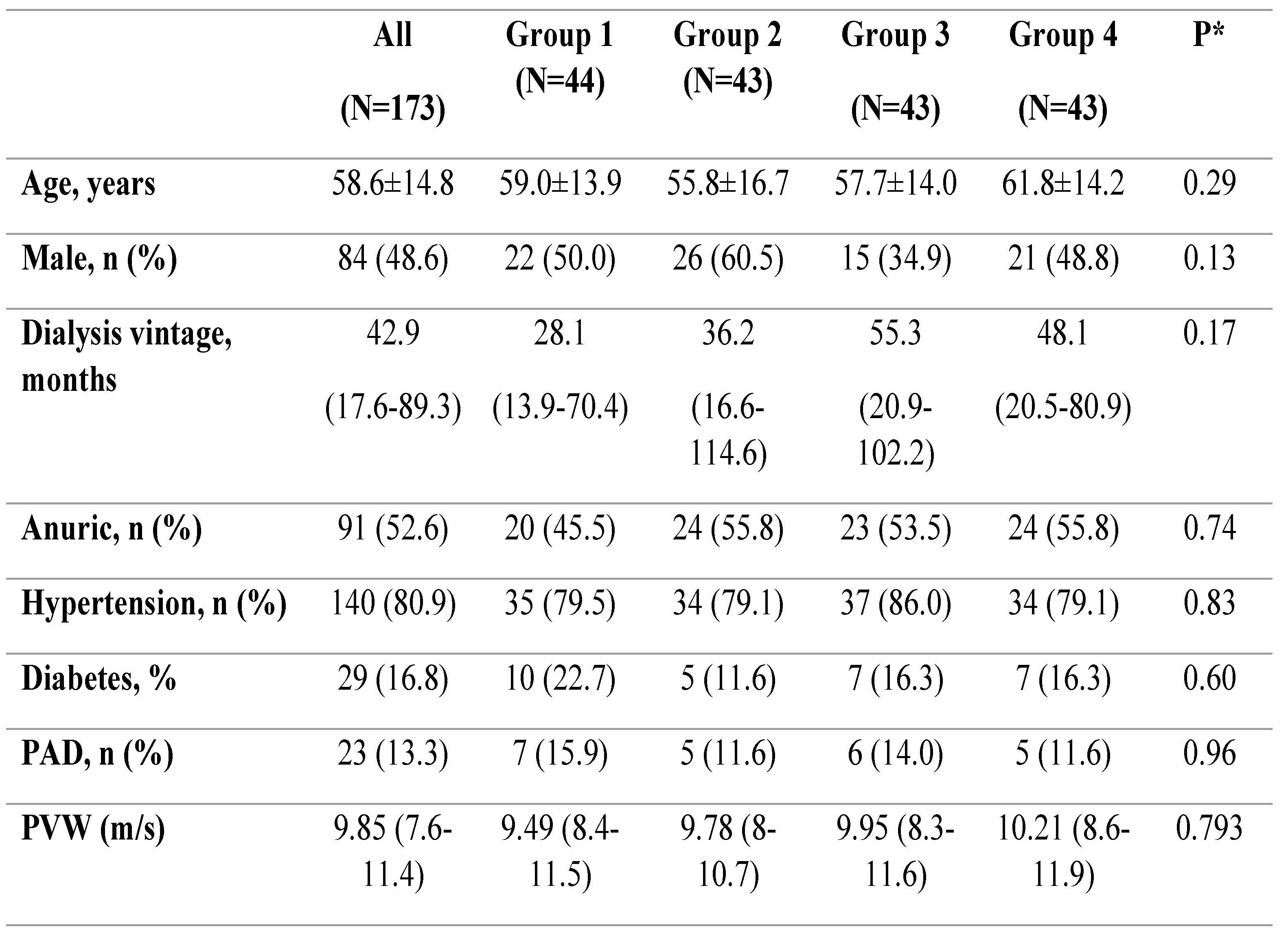
173 patients were analysed in this follow-up cohort. The mean baseline age was 58.6± 14.8 years; 48.6% were male, and 16.2% had diabetes mellitus. The median dialysis vintage was 42.9 months (IQR 17.6–89.3). The median levels of Gal-3 and NT-proBNP were 28.1 (ng/mL, IQR: 18.7-40.4ng/mL) and 4234 pg/mL (IQR: 1826.5-11.581pg/mL), respectively. The patients were divided into four groups based on median Gal-3 and NT-proBNP levels:
Group 1: low Gal-3 / low NT-proBNP (n = 44)
Group 2: high Gal-3 / low NT-proBNP (n = 43)
Group 3: low Gal-3 / high NT-proBNP (n = 43)
Group 4: high Gal-3 / high NT-proBNP (n = 43)
Baseline demographic, clinical, biological and vascular characteristics of the entire population are presented in
Table 1.
3.2. Primary Outcome – All-Cause Mortality
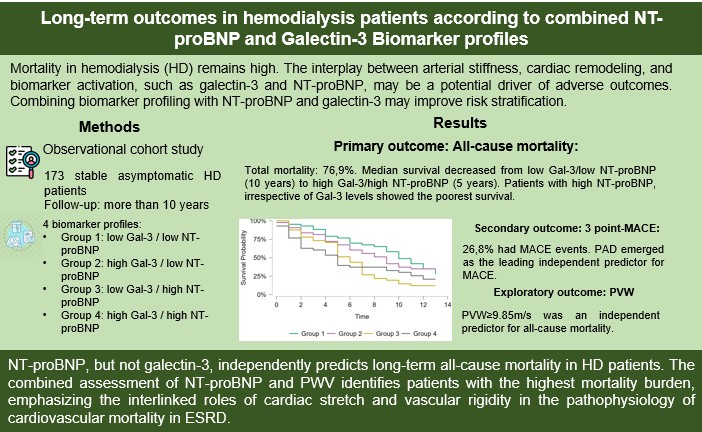
Over a median follow-up of more than 10 years, 76.9% of patients died. Among patients with known causes of death, 21.1% died from cardiovascular causes, 21.1% from sepsis, and 22.6% from other causes (pulmonary embolism, terminal illness, or intracerebral hemorrhage). Sudden death occurred in 31,6% of all recorded deaths, according to the electronic dialysis database. The cause of death remained undetermined in four patients.
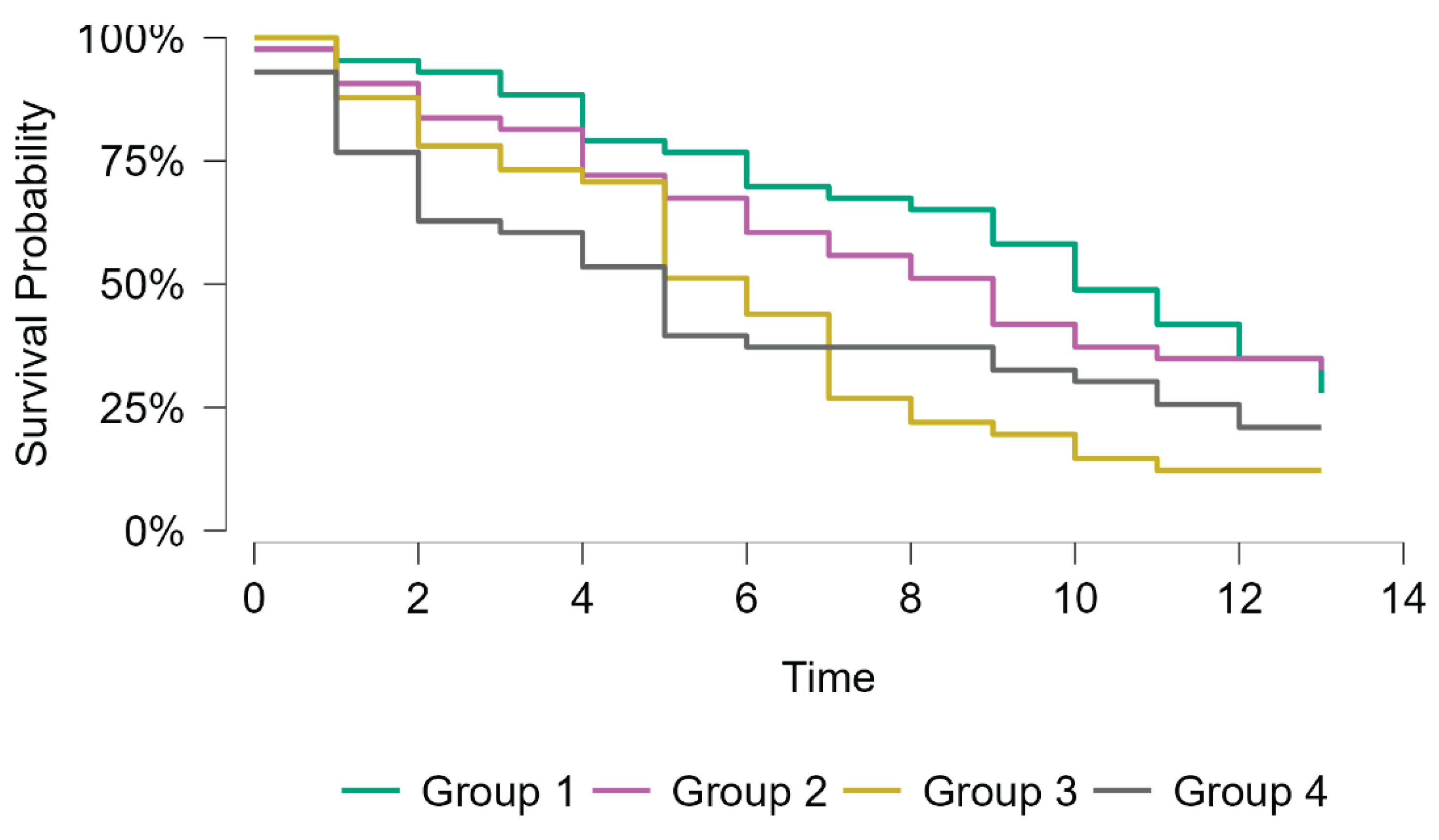
Kaplan-Meier survival analysis for all-cause mortality showed significant differences among the four groups (Log-rank χ² = 10.6, p = 0.014). Median survival progressively decreased from Group 1 (10 years) to Group four (five years). Patients with high levels of NT-proBNP (groups three and four) demonstrated the poorest survival rate compared to those with low levels of NT-proBNP (groups one and two). Group 4 had the shortest median survival time (5 years, 95% CI, 2-10), followed by group 3 (6 years, 95% CI, 9-13). The survival curves diverged early during follow-up, with the greatest separation observed after 5 years (
Figure 1).
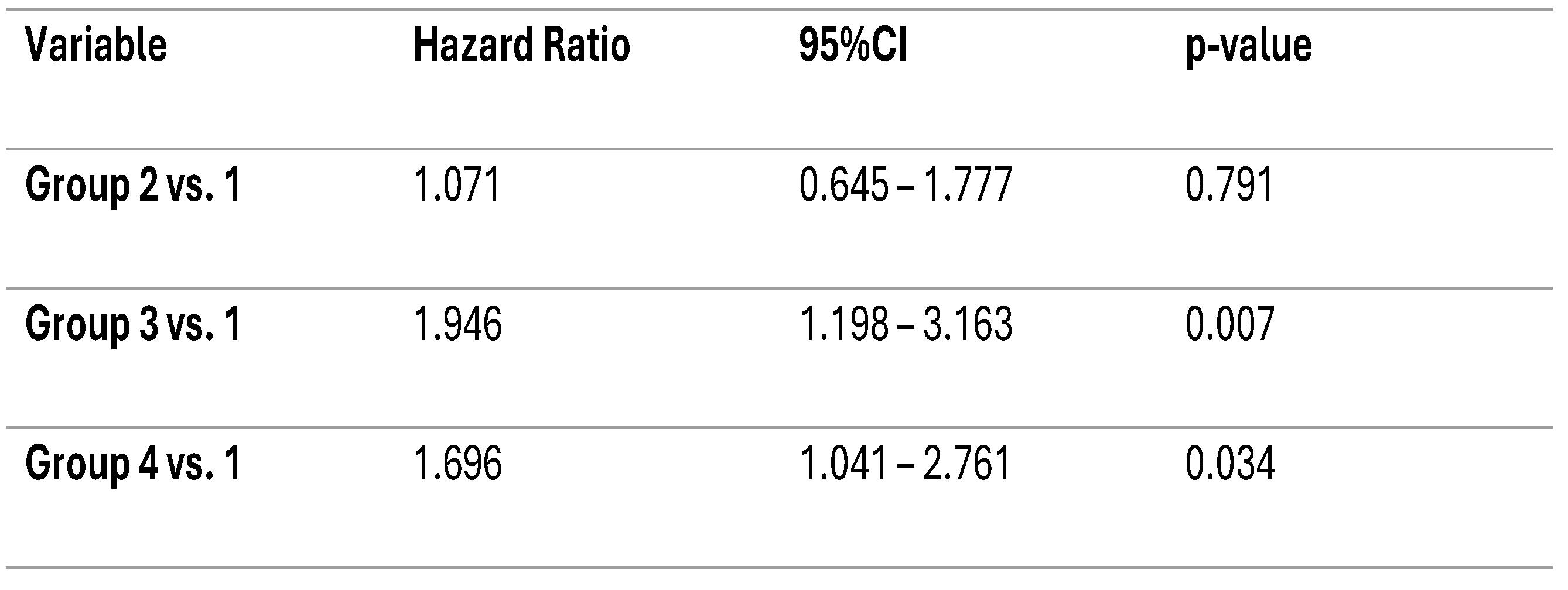
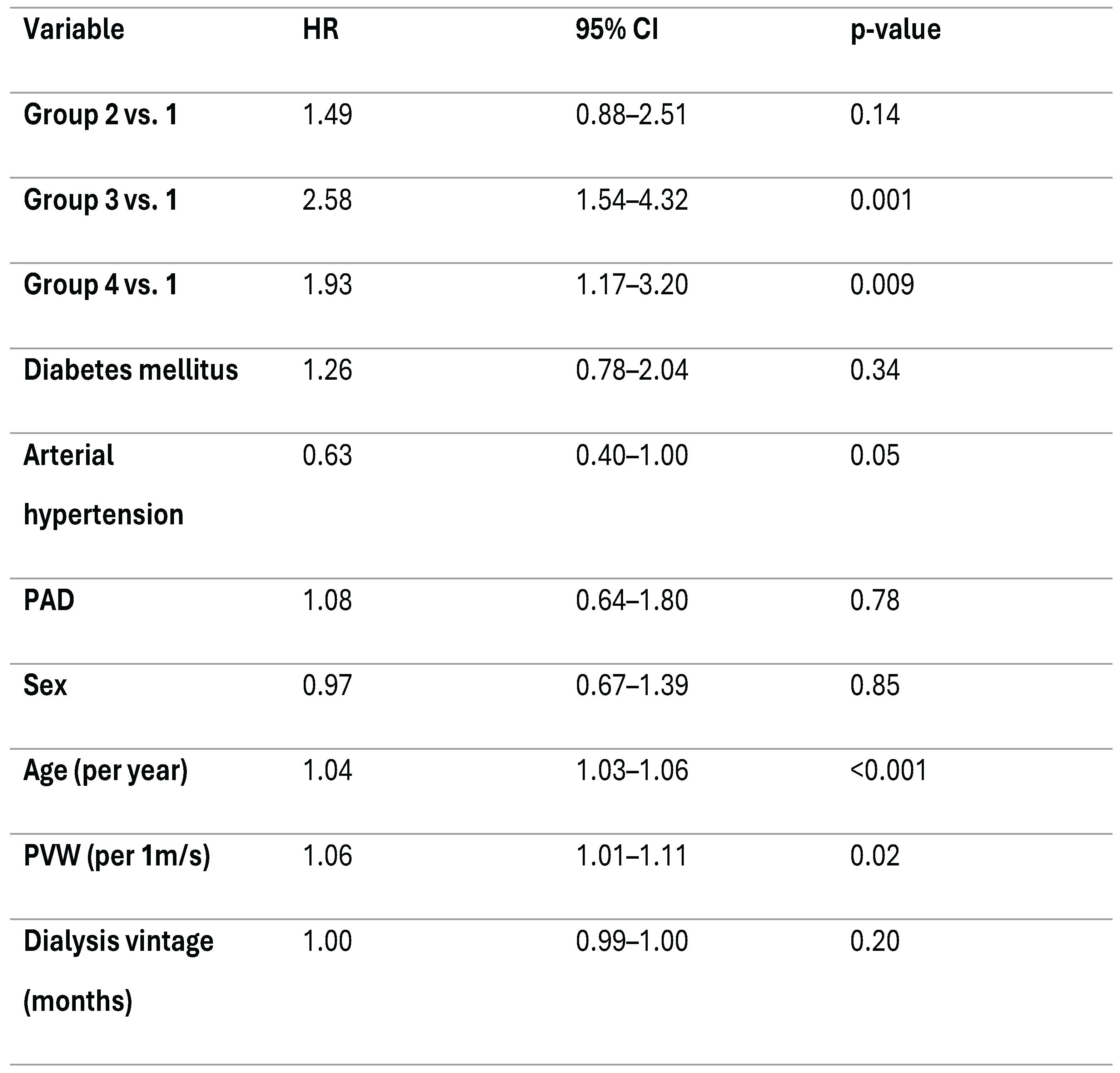
In the unadjusted Cox Regression model, group one was used as the reference category. Compared with group one, patients in groups three and four had a higher risk of all-cause mortality (HR = 1.95, 95% CI 1.20–3.16, p = 0.007; and HR = 1.70, 95% CI 1.04–2.76, p = 0.034, respectively). Group two did not differ as much from the reference group (HR = 1.07, 95% CI 0.65–1.78, p = 0.79). These results suggest that a profile characterized by high NT-proBNP levels, alone or in combination with high Gal-3 levels, is associated with increased long-term mortality in hemodialysis patients (
Table 2).
Next, a multivariable Cox proportional hazards model was performed to identify independent predictors of all-cause mortality. The model was adjusted for age, sex, presence of diabetes mellitus, arterial hypertension, peripheral artery disease, pulse wave velocity and dialysis vintage (months). After adjustment, both Group 3 and Group 4 remained independently associated with mortality (HR 2.58 [1.54–4.32], p < 0.001; HR 1.93 [1.17–3.20], p = 0.011). Group 2 did not differ significantly from the reference.
Among the covariates, both age and PVW were independently associated with higher mortality risk. Each one-year increase in age was associated with a 4% higher risk of death (HR 1.04 [95% CI 1.03–1.06], p < 0.001). On the other hand, each 1m/s increase in PVW corresponded to a 6% increase in mortality risk (HR 1.06 [95% CI 1.01–1.11], p = 0.019). Arterial hypertension showed a borderline protective effect (HR 0.63 [95% CI 0.40–1.00], p = 0.05). The other covariates (diabetes mellitus, peripheral artery disease, sex and dialysis vintage) were not associated with an increase in mortality after adjustment (
Table 3).
3.2.1. Sensitivity Analysis
Proportionality of hazards were verified using scaled Schoenfeld residuals. The global test revealed a mild departure from proportionality (χ² = 19.37, df = 10, p = 0.036), primarily driven by the variables age (p = 0.006) and group (p = 0.029). All other covariates met the proportional hazards assumption (P>0.05). The overall pattern of residuals did not indicate major time-dependent effects, and the assumption of proportional hazards was considered reasonably met.
3.3. Secondary Outcome – MACE
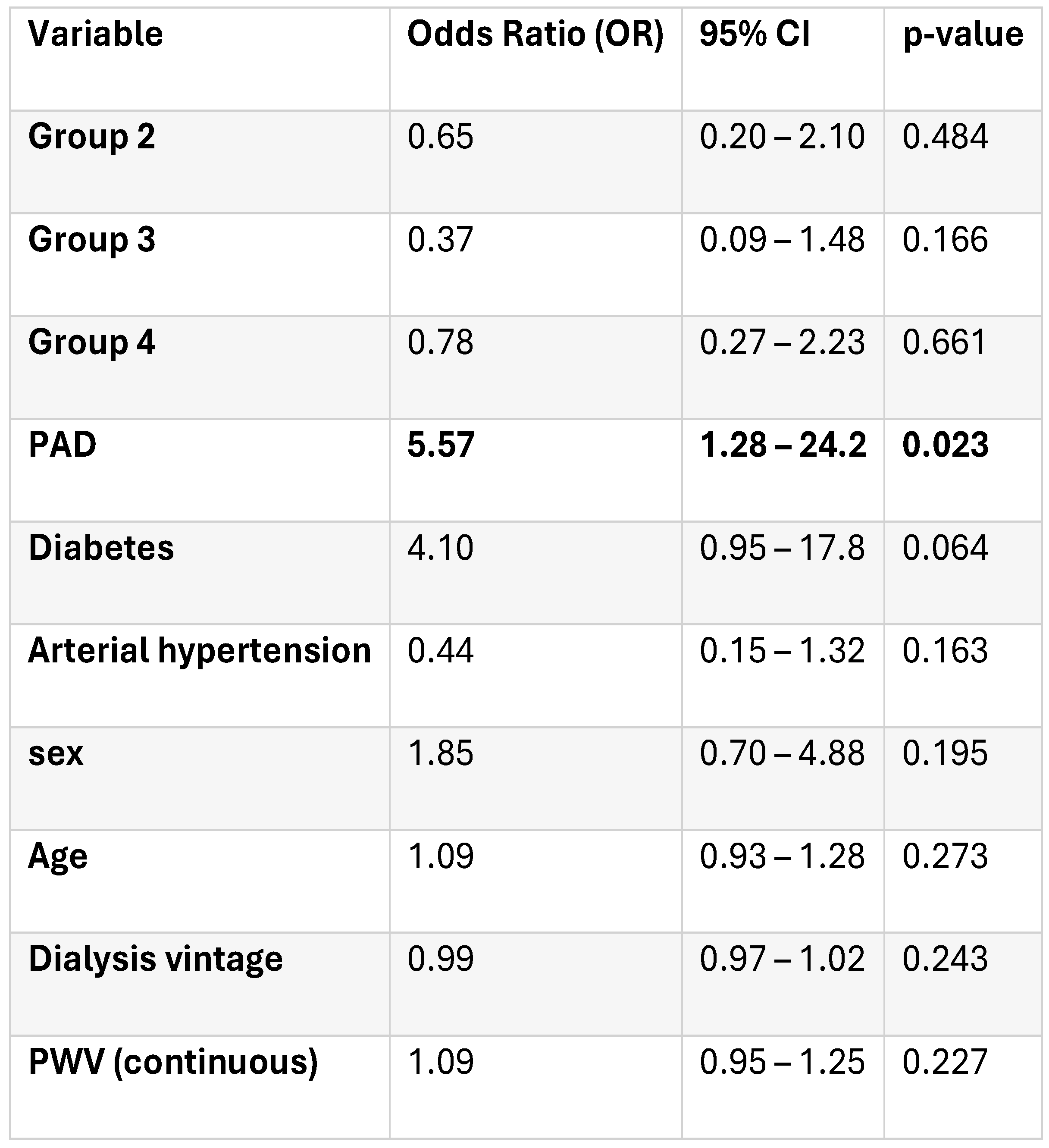
For MACE analysis, data were available for 127 patients after excluding those with sudden or undetermined death. During follow-up, 34 MACE events (26.8%) were recorded: 79.4% cardiovascular deaths, 14.7% non-fatal myocardial infarctions, and 5.9% non-fatal strokes. In the unadjusted logistic regression model excluding patients with sudden death, no statistically significant differences in MACE risk were observed across the predefined groups (p=0.71). Compared to group 1, the odds ratios for MACE were 0.70 (95% CI 0.23–2.10, p = 0.53) for Group 2, 0.53 (95% CI 0.16–1.72, p = 0.28) for Group 3, and 0.88 (95% CI 0.30–2.58, p=0.8). Model fit indices indicated no improvement in predictive power compared to the null model (Δχ² = 1.37, p = 0.71; Nagelkerke R² = 0.016).
To test the robustness of our findings, we repeated the multivariate logistic regression including patients who experienced sudden death and in patients for whom we did not know the cause of death (n = 173). The results remained directionally consistent with the primary model, although none of the predictors reached statistical significance (global model p = 0.42, Nagelkerke R² = 0.09). Peripheral artery disease and diabetes remained positively associated with MACE risk, whereas PWV and biomarker-defined groups showed no significant association.
After adjusting for demographic, vascular, and dialysis-related factors, peripheral artery disease emerged as the leading independent predictor of MACE, with patients showing a 5.5-fold higher risk compared to those without PAD. Diabetes showed a borderline significant trend. No independent association was found for the biomarker-defined groups or PVW (
Table 4).
3.4. Exploratory Outcomes
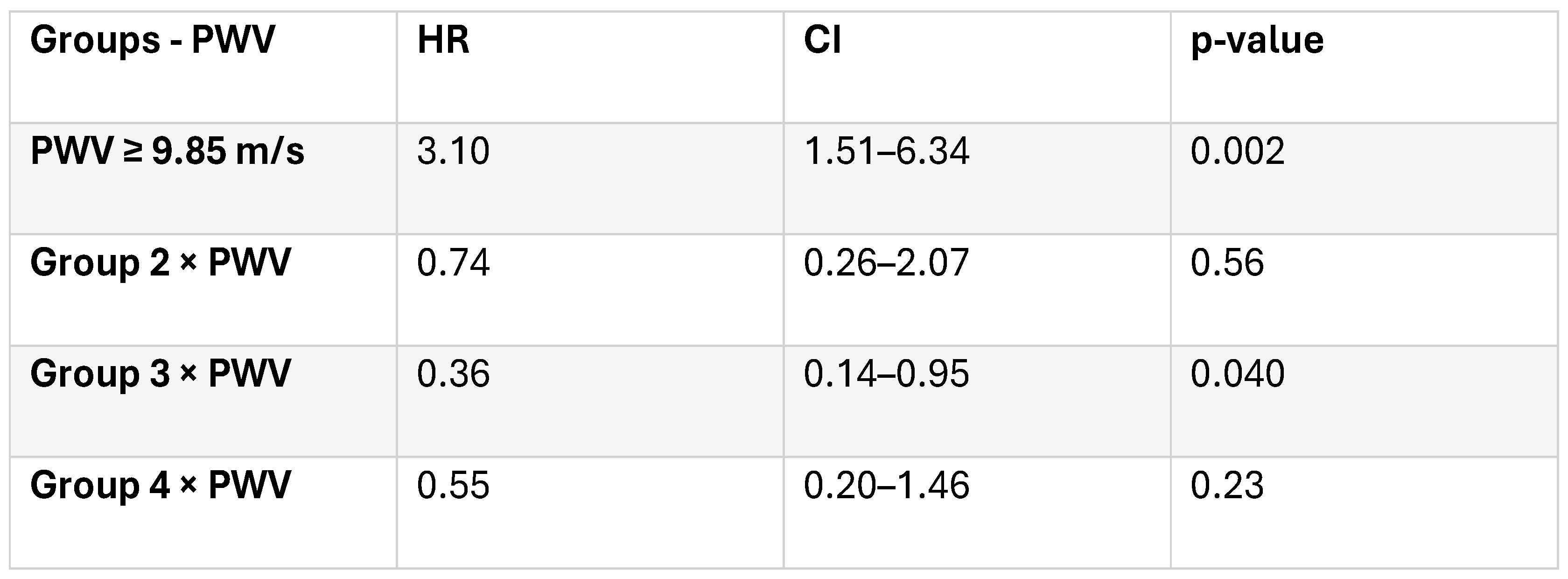
A semi-parametric Cox proportional hazards model including group, PWV, and their interaction was fitted to assess the differential impact of arterial stiffness across biomarker-defined subgroups. The analysis included 170 patients and 130 events. Model comparison showed a slight improvement in fit (AIC = 1179.18 vs. 1179.80). The global proportional hazards test revealed mild deviation (χ² = 19.37, df = 10, p = 0.036), but the model was considered acceptable.
In the multivariate Cox model including the defined groups and PVW and their interaction, results indicated that a PVW ≥ 9.85 m/s was independently associated with all-cause mortality (HR 3.1, 95% CI 0.2-1.46, p=0.002). A significant interaction between Group 3 and PWV (p = 0.04) was observed, suggesting that the effect of arterial stiffness on mortality differed across biomarker-defined groups. In Group 4 (both biomarkers elevated), mortality was already high irrespective of arterial stiffness (
Table 5).
4. Discussion
In this long-standing study, the biomarker profile that included elevated baseline NT-proBNP levels were significantly associated with all-cause mortality over a follow-up period exceeding 10 years, irrespective of galectin-3. In addition, arterial stiffness, as reflected by higher PVW, was an independent predictor of mortality, with its impact particularly pronounced in patients with high NT-proBNP and low galectin-3 levels. These findings underline the prognostic value of mixing cardiac and vascular markers in the risk assessment of HD patients.
This study provides one of the most extended follow-up periods reported in a dialysis cohort, enabling a reliable assessment of long-term outcomes. Patients were categorized using both clinical and biomarker-based parameters, providing a comprehensive evaluation of cardiovascular and mortality risk beyond traditional factors. The use of biomarker-defined subgroups (NT-proBNP and galectin-3) denotes a contemporary approach to catch heterogeneous cardiovascular phenotypes in hemodialysis.
In our previous 3-year follow-up study, NT-proBNP and galectin-3 were both linked with CV outcomes in stable, asymptomatic HD patients [
7]. The present analysis reinforces the notion that NT-proBNP remains a robust predictor even after a decade, whereas galectin-3 loses independent significance over time. This suggests that NT-proBNP may replicate a more stable pathophysiologic signal, catching both volume and pressure overload, as well as subclinical myocardial strain—hallmarks of a poor long-term prognosis in ESKD. In contrast, galectin-3 seems more closely connected to intermediate processes, such as inflammation and fibrosis, which may fluctuate with dialysis-related or systemic factors [
8].
Previous meta-analyses in CKD and HD cohorts have described varying links between galectin-3 and mortality. Zhang et al. [
6] found that elevated galectin-3 was associated with all-cause mortality in CKD, but its prognostic value in HD remained uncertain. More recent signals, including the meta-analysis by Bellos et al. [
9], have confirmed that galectin-3 levels above 30 ng/mL are associated with a higher mortality risk. However, this connection was frailer in HD than in pre-dialysis CKD. Our findings are consistent with these observations and indicate that, within a long-term HD population, galectin-3 alone may not provide additional predictive value beyond NT-proBNP.
NT-proBNP and galectin-3 reveal different but complementary biological pathways. NT-proBNP mainly reflects myocardial stretch, cavity pressure, and volume overload, although galectin-3 is involved in macrophage activation, myocardial fibrosis, and systemic inflammation [
10]. In dialysis patients, chronic exposure to fluid overload, uremic toxins, and repetitive myocardial ischemia-reperfusion episodes cause both cardiac remodeling and vascular stiffening [
11]. Galectin-3 seems more faithfully linked to intermediate processes, such as inflammation and fibrosis, which may vary with dialysis-related or systemic aspects. Galectin-3 may be a mediator rather than a marker of pathology—its elevation may lead to tissue remodeling but not inevitably predict long-term outcomes once structural cardiac damage is recognized [
12]. This hypothesis aligns with mechanistic studies showing that galectin-3 inhibition in animal models reduces fibrosis but does not reverse established structural abnormalities [
13].
Arterial stiffness independently predicted mortality and amplified the risk conferred by raised NT-proBNP. This finding supports the notion that cardiac and vascular dysfunction act synergistically to influence outcomes in ESKD. Their interface advocates that vascular stiffness may exacerbate cardiac stress by augmenting afterload, thus supporting a vicious cycle of myocardial strain and maladaptive remodeling [
14].
PWV has been constantly associated with mortality in HD, but its prognostic integration with biomarkers continues to be limited [
15]. Our results establish that joining PWV with NT-proBNP recognizes patients at particularly high long-term risk. This tactic could improve risk stratification outside conventional clinical variables, especially in asymptomatic individuals without overt cardiovascular disease.
These results have some possible clinical implications. NT-proBNP remains a strong and inexpensive predictor of long-term outcomes in HD, and its assessment could help identify patients who might benefit from enhanced cardiovascular surveillance or more stringent volume management. The additive prognostic role of PWV supports the use of non-invasive vascular evaluation in HD care. Merging NT-proBNP and PWV may provide a simple, clinically applicable model for personalized risk stratification. In contrast, the role of galectin-3 as a mediator of myocardial fibrosis and inflammation warrants further exploration, particularly in combination with other markers and in relation to temporal changes rather than baseline levels.
Limitations of our study include: the cohort size was modest, and biomarker measurements were obtained at a single baseline time point. Temporal changes in NT-proBNP, galectin-3, and PWV were unavailable, limiting the assessment of dynamic risk trajectories. The high proportion of sudden deaths in our cohort may have led to an underestimation of cardiovascular-specific mortality. Although the study had an unusually long follow-up and minimal loss to observation, residual confounding by unmeasured factors cannot be excluded. Finally, the results are derived from a single geographic region and may not generalize to other populations or dialysis modalities.
5. Conclusions
In summary, this study suggests that NT-proBNP, but not galectin-3, predicts long-term all-cause mortality in HD patients, while arterial stiffness remains a strong, additive determinant of risk. The combined assessment of NT-proBNP and PWV identifies patients with the highest mortality burden, emphasizing the interlinked roles of cardiac stretch and vascular rigidity in the pathophysiology of cardiovascular mortality in end-stage kidney disease.
Author Contributions
S A-E: conceptualization, methodology, writing, manuscript review, statistical analysis, data processing, AC: manuscript review, conceptualization, methodology, AC: manuscript review, methodology, data processing, data interpretation, MO: data processing, statistical analysis, AM, SH: data processing, data interpretation, writing, GD: writing, reference checking, manuscript review, LV: conceptualization, methodology, writing, supervision, manuscript review.
Funding: Please add: “This research received no external funding” or “This research was funded by NAME OF FUNDER, grant number XXX” and “The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at
https://search.crossref.org/funding. Any errors may affect your future funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Hospital “Dr. C. I. Parhon”.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CKD |
Chronic kidney disease |
| CV |
Cardiovascular |
| Gal-3 |
Galectin-3 |
| NT-proBNP |
N-terminal pro-B-type natriuretic peptide |
| ESRD |
End-stage renal disease |
| PVW |
Pulse wave velocity |
| HD |
hemodialysis |
References
- Qin, K.; Qing, J.; Wang, Q.; Li, Y. Epidemiological shifts in chronic kidney disease: a 30-year global and regional assessment. BMC Public Health 2024, 24(no. 1), 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C. P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022, 12(no. 1), 7. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease Pathophysiological Insights and Therapeutic Options https://doi.org/10.1161/CIRCULATIONAHA.120.050686/ASSET/A0E7BD90-D49C-40B3-BE21-A29DB8F19F7C/ASSETS/IMAGES/LARGE/CIRCULATIONAHA.120.050686.FIG07.JPG. Circulation 2021, 143(no. 11), 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. H.; Noh, H. M.; Song, H. J.; Lee, S.; Kim, S. G.; Kim, J. K. Mediating effect of vascular calcification in galectin-3-related mortality in hemodialysis patients. Scientific Reports 2024, 14(no. 1), 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tınazlı, M.; Bakhtiyarova, G. Galectins: An Amazing Marker and a Potential Therapeutic Target Abstract. 2025. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Yang, H.; Li, J. Prognostic impact of galectin-3 in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol 2019, 51(no. 6), 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Voroneanu, L. Prospective Validation of a Screening Biomarker Approach Combining Amino-Terminal Pro-Brain Natriuretic Peptide With Galectin-3 Predicts Death and Cardiovascular Events in Asymptomatic Hemodialysis Patients. Angiology 2018, 69(no. 5), 449–455. [Google Scholar] [CrossRef] [PubMed]
- Li, L. C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther 2014, 351(no. 2), 336–343. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Marinaki, S.; Lagiou, P.; Benetou, V. Association of serum galectin-3 levels with mortality and cardiovascular disease outcomes in hemodialysis patients: a systematic review and dose–response meta-analysis. Int Urol Nephrol 2024, 56(no. 8), 2755–2767. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2021, 12(no. 1). [Google Scholar] [CrossRef] [PubMed]
- Curaj, A. Cardiovascular Consequences of Uremic Metabolites: an Overview of the Involved Signaling Pathways. Circ Res 2024, 134(no. 5), 592–613. [Google Scholar] [CrossRef] [PubMed]
- Martuszewski, *!!! REPLACE !!!*; Paluszkiewicz, P.; Poręba, R.; Gać, P. Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks. Current Issues in Molecular Biology 2025 2025, 47(no. 5), 332. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W. C.; Silljé, H. H. W.; Ho, J. E.; Liu, F. T.; de Boer, R. A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8(no. 3), 593. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J. A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019, 74(no. 9), 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A. P.; Pannier, B.; Marchais, S. J.; Safar, M. E.; London, G. M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99(no. 18), 2434–2439. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).