Submitted:
13 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
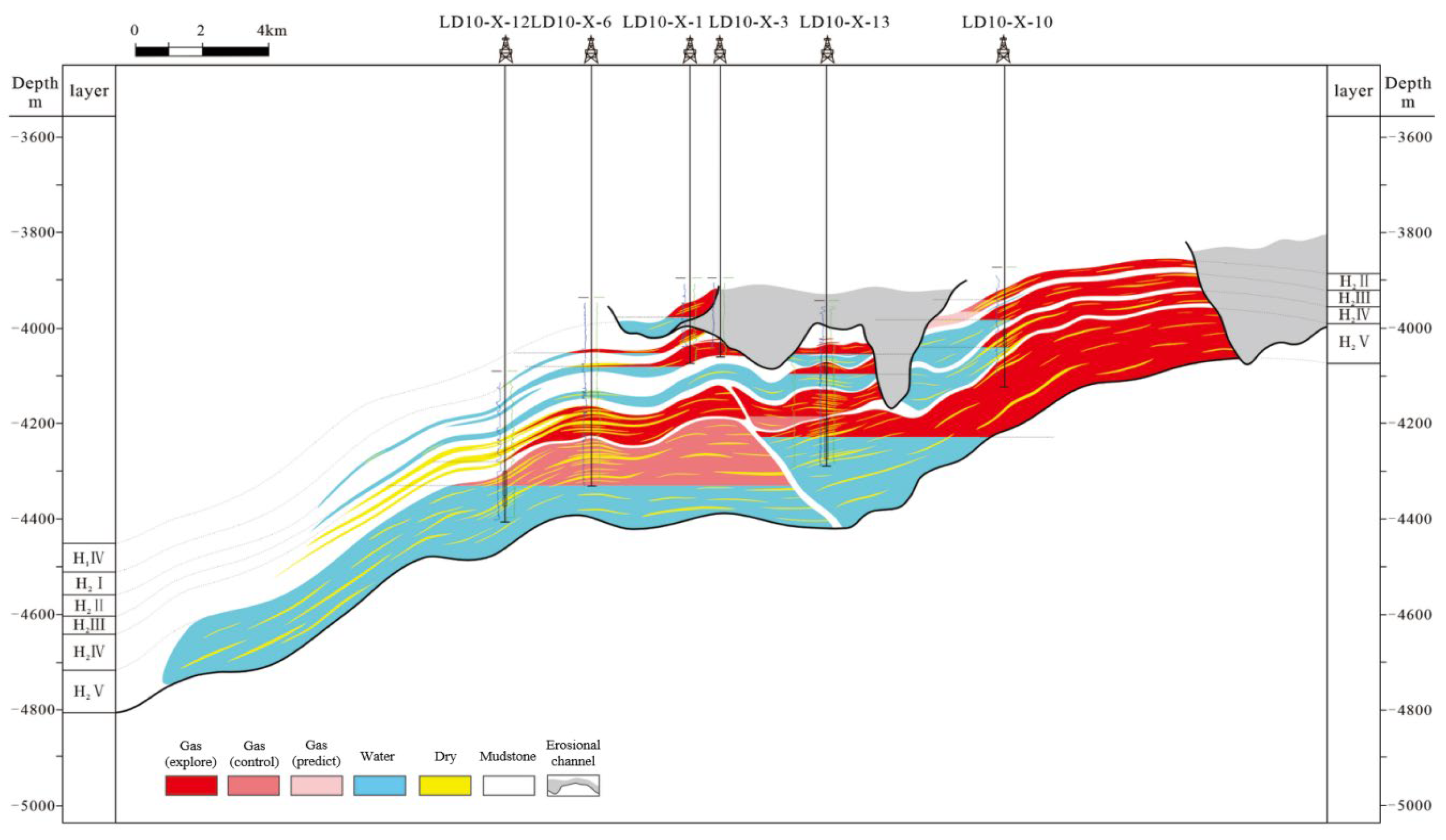
2. Geological Characteristics of LD10-X Gas Field
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Measurement of Solubility of CO2
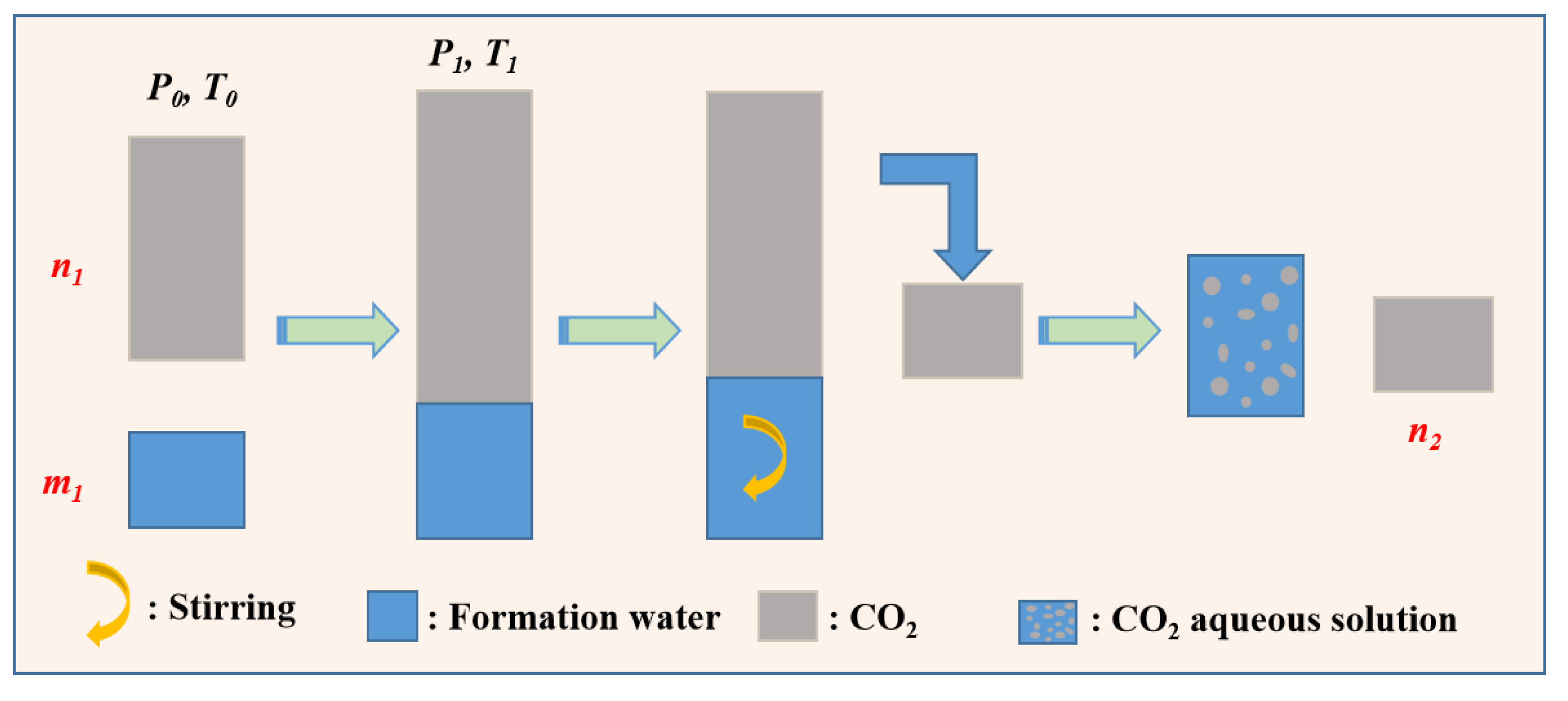
3.2.2. Measurement of Solubility of CH4
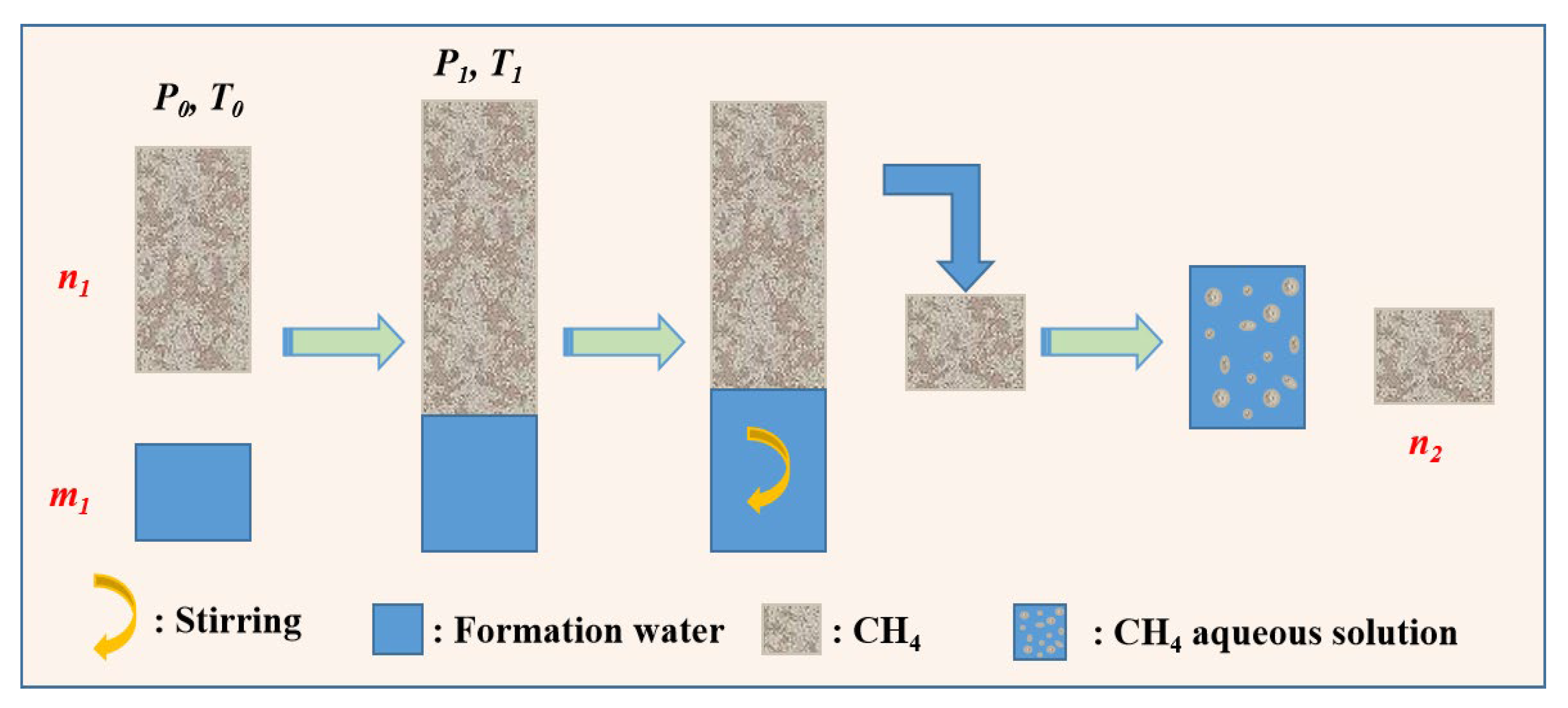
3.2.3. Measurement of Exsolution of CO2
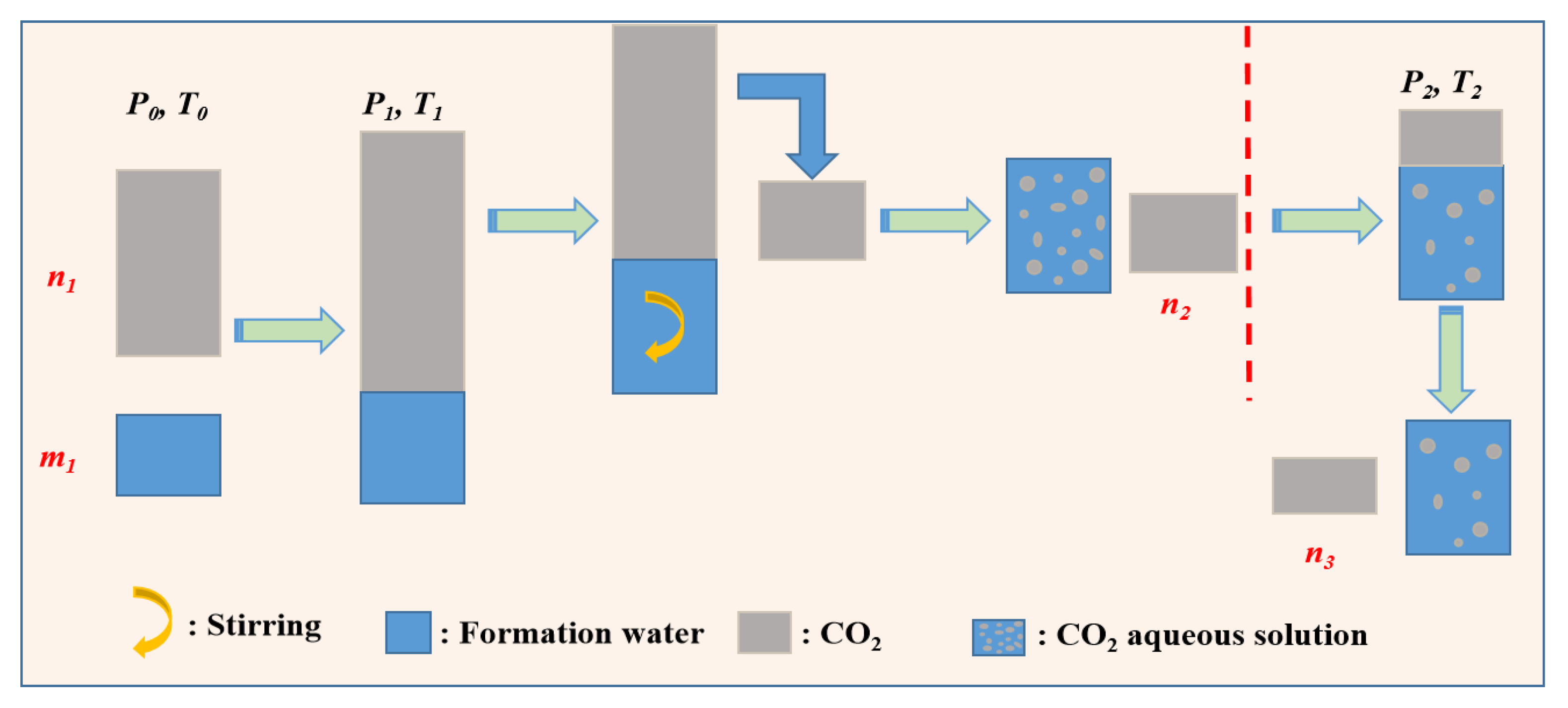
3.2.4. Measurement of Exsolution of CH4
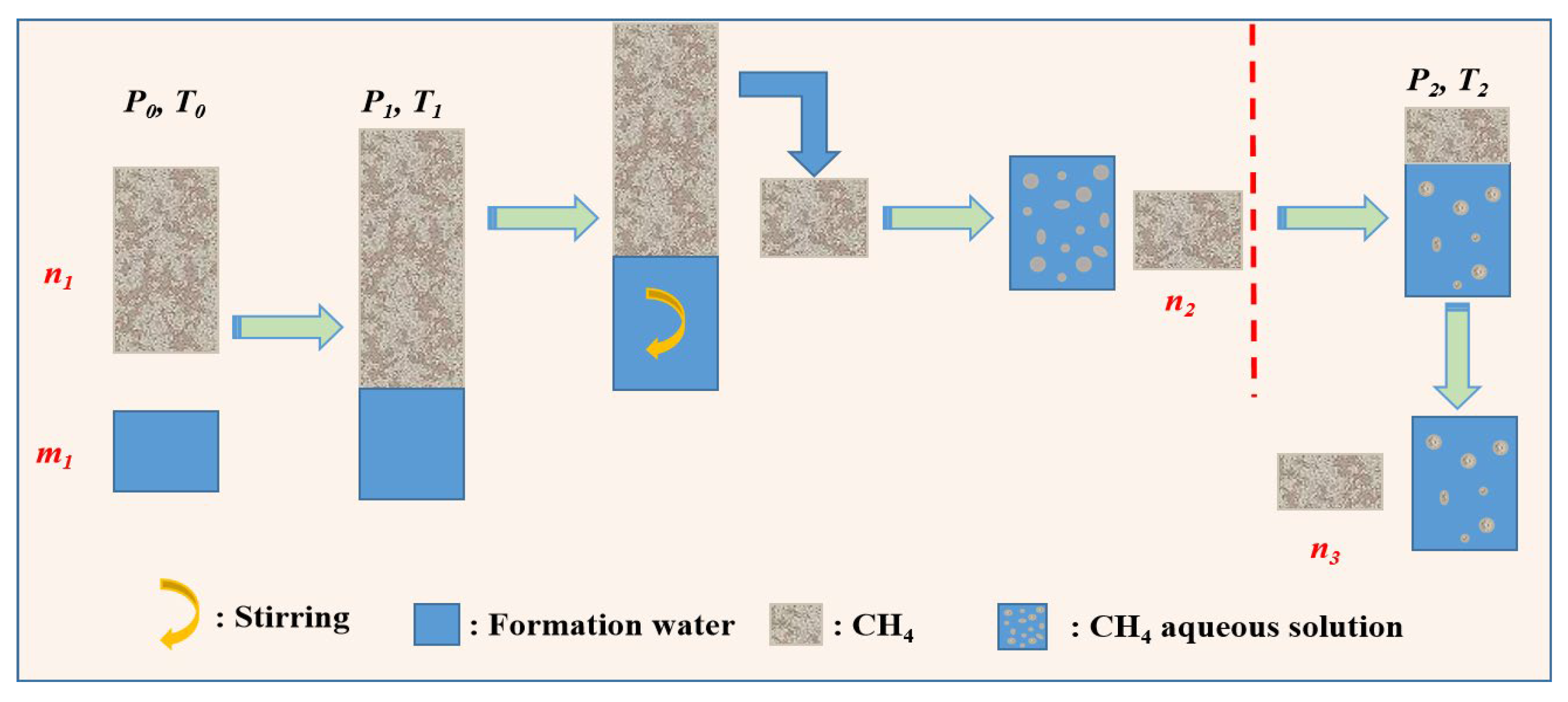
4. Results and Discussion
4.1. Study on fluid Phase Characteristics of LD10-X Gas Field
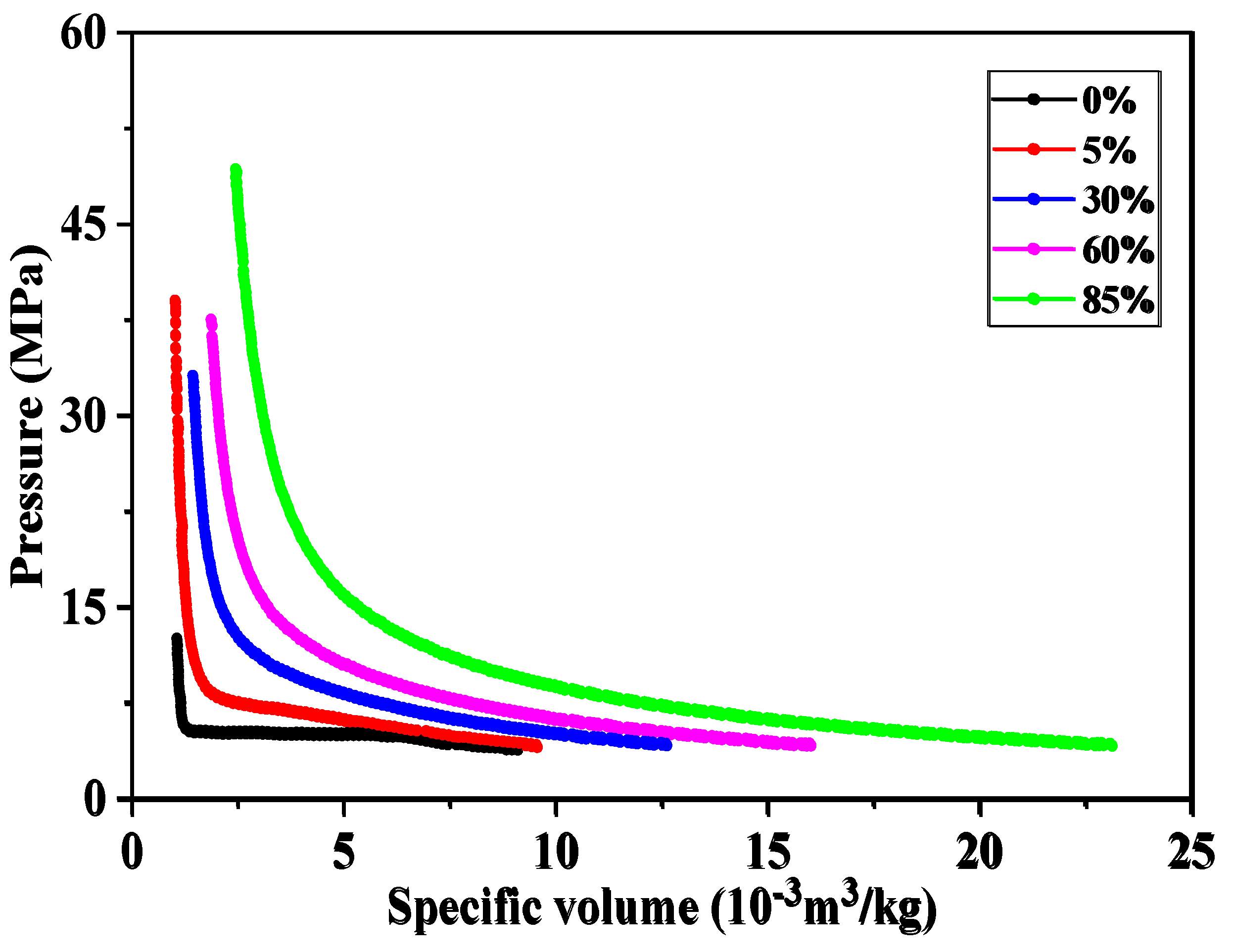
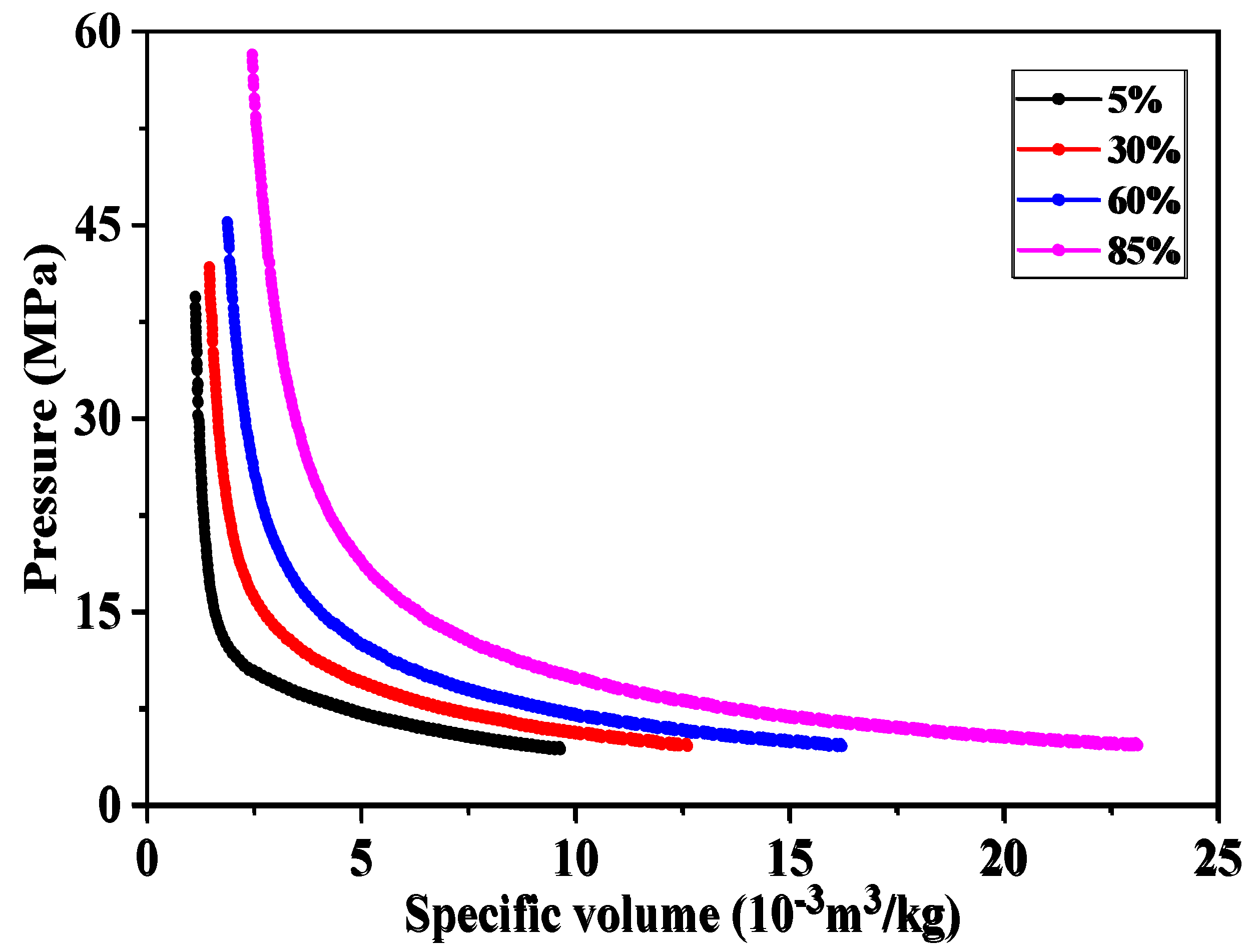
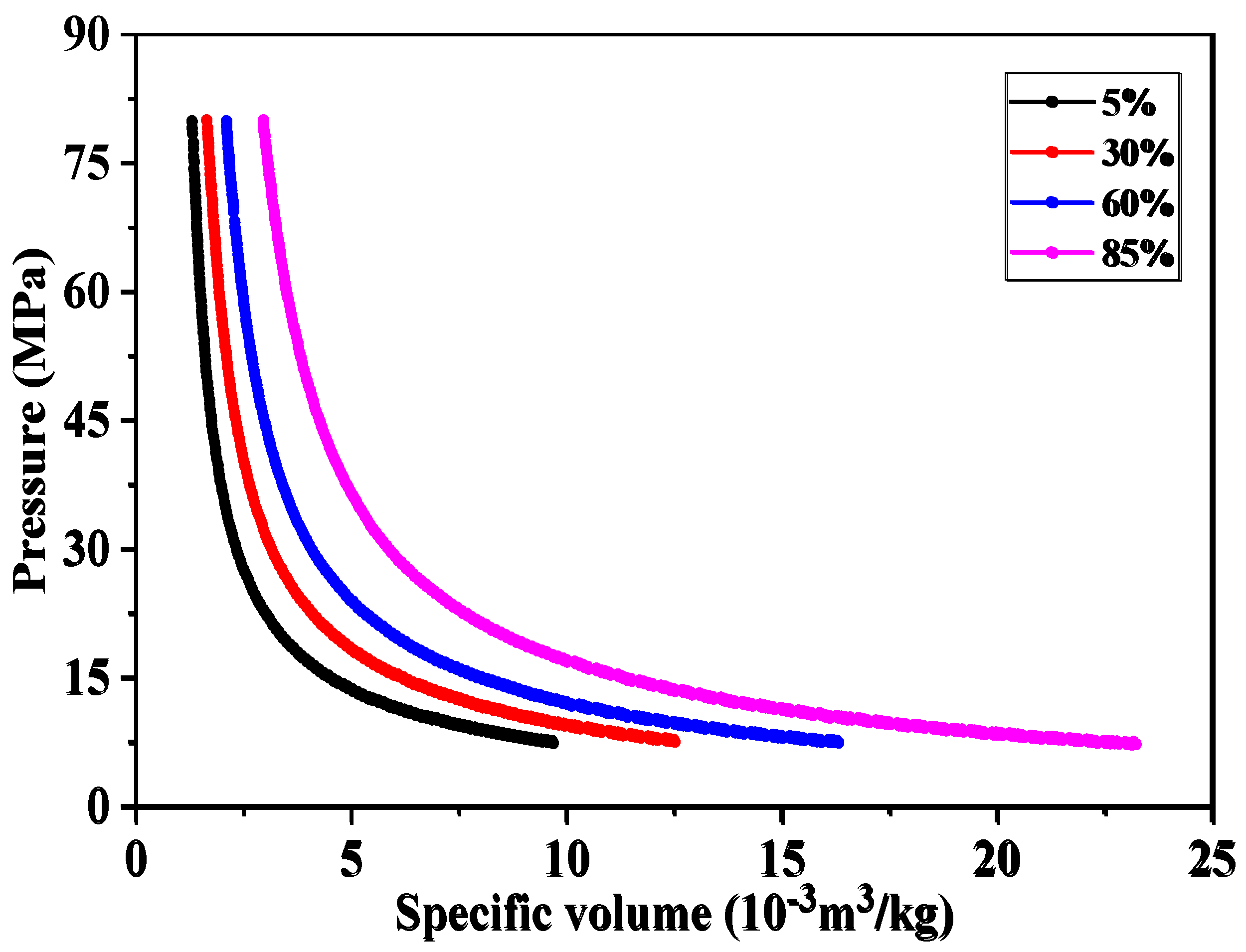
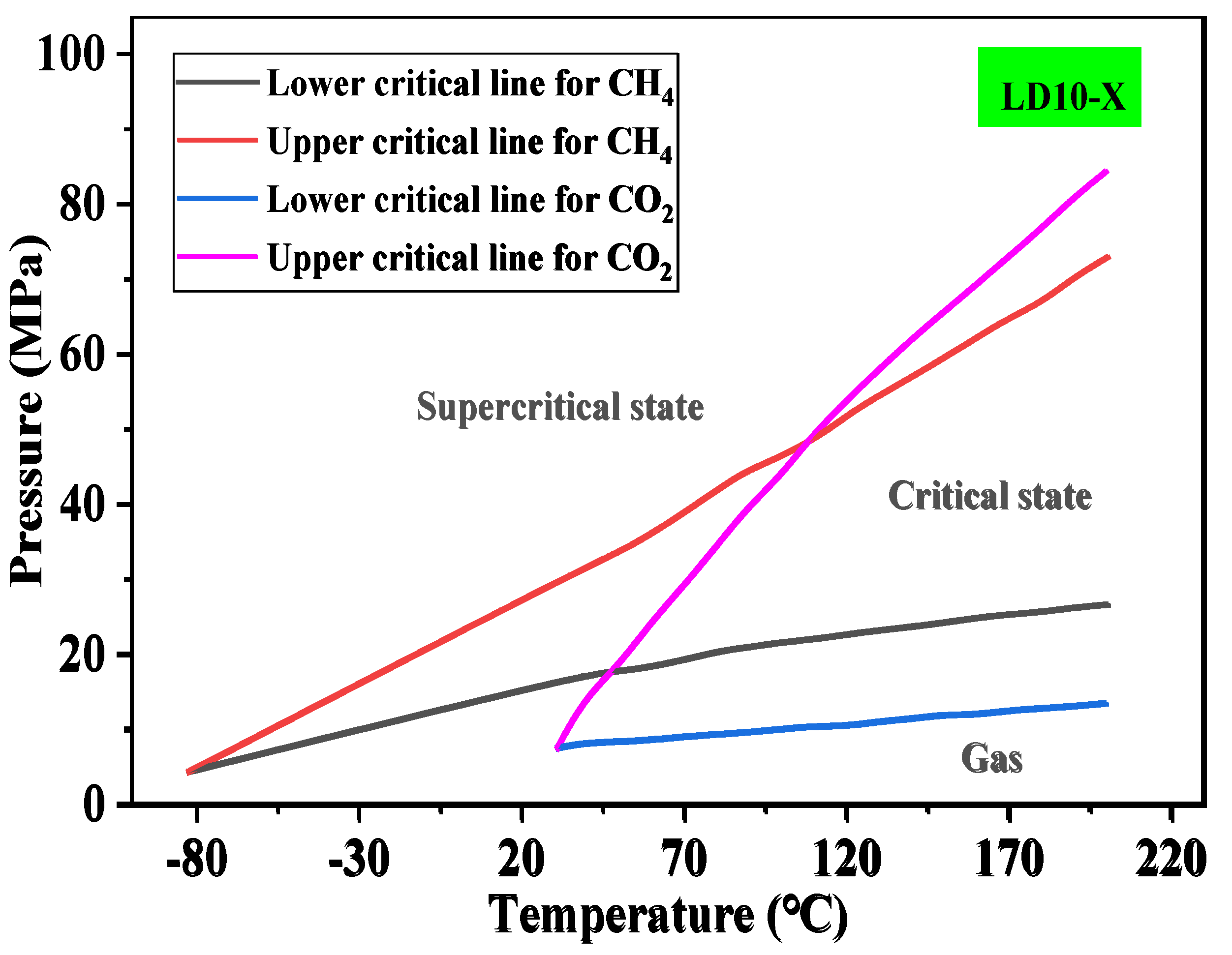
4.2. Study on the Solubility Law of CH4 and CO2 in Formation Water
4.2.1. The Effect of CO2 and CH4 Mixing Ratio on Solubility
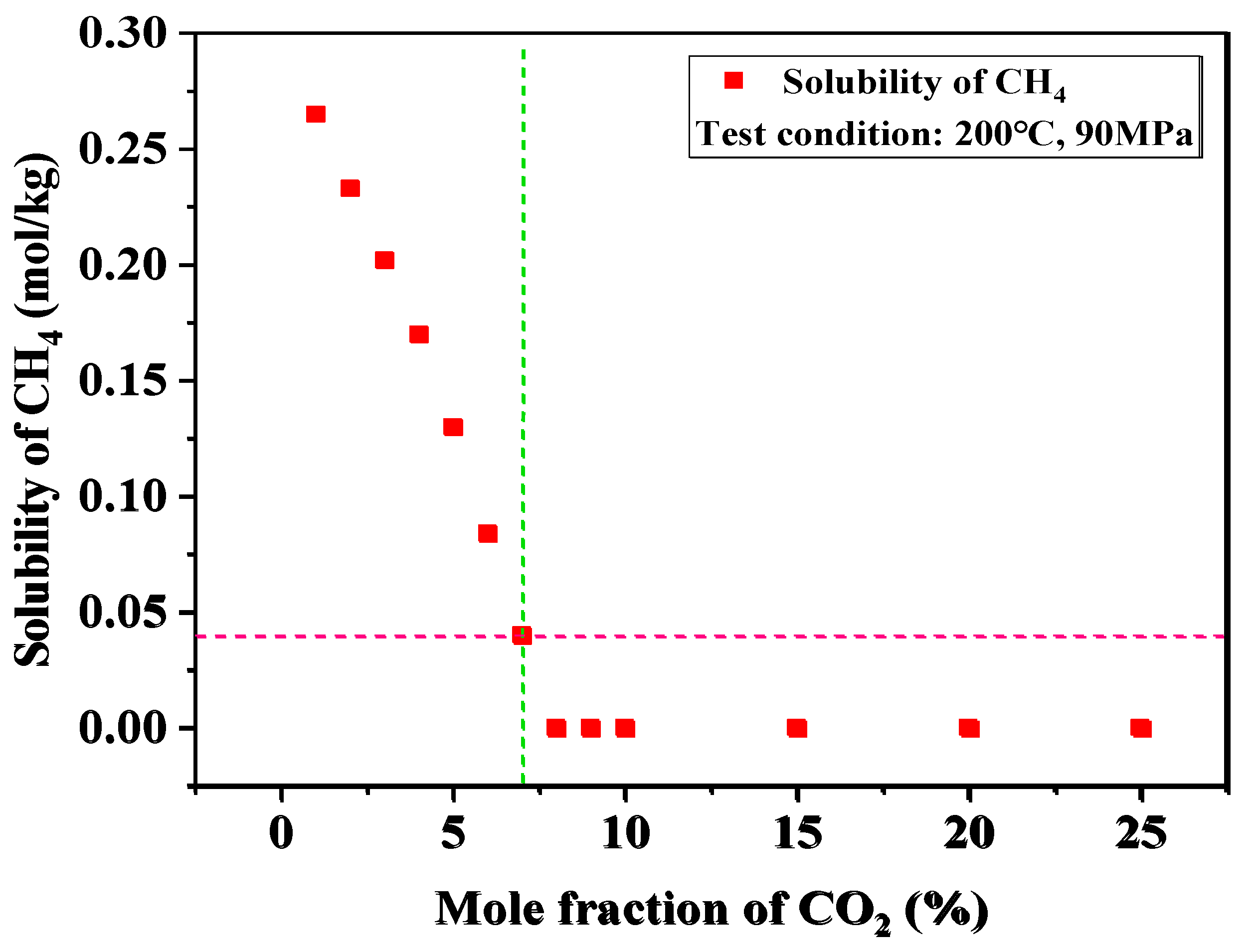
4.2.2. The Effect of CO2 and CH4 Solubility Sequence on Solubility
4.2.3. The Solubility of CO2 and CH4 with Temperature and Pressure
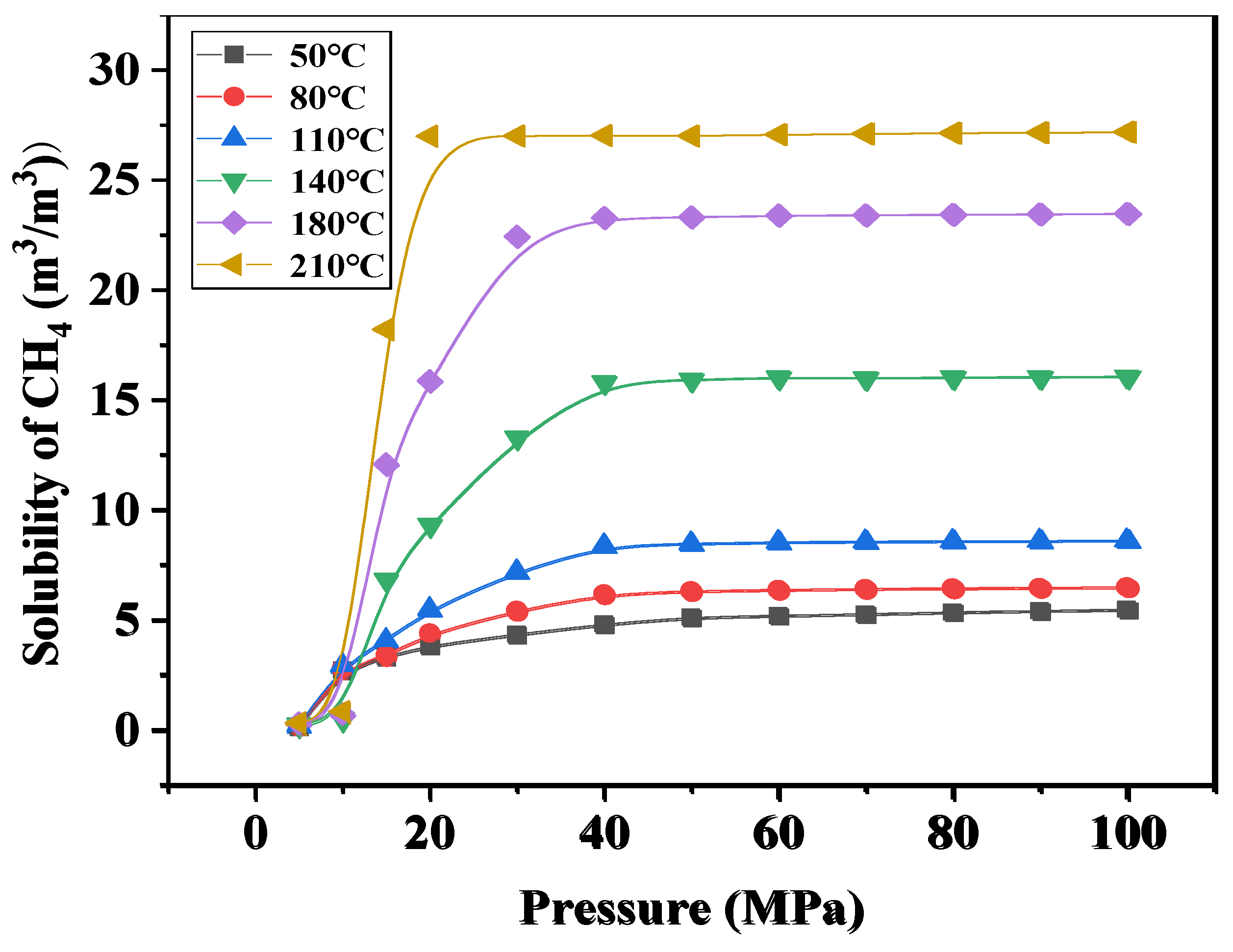
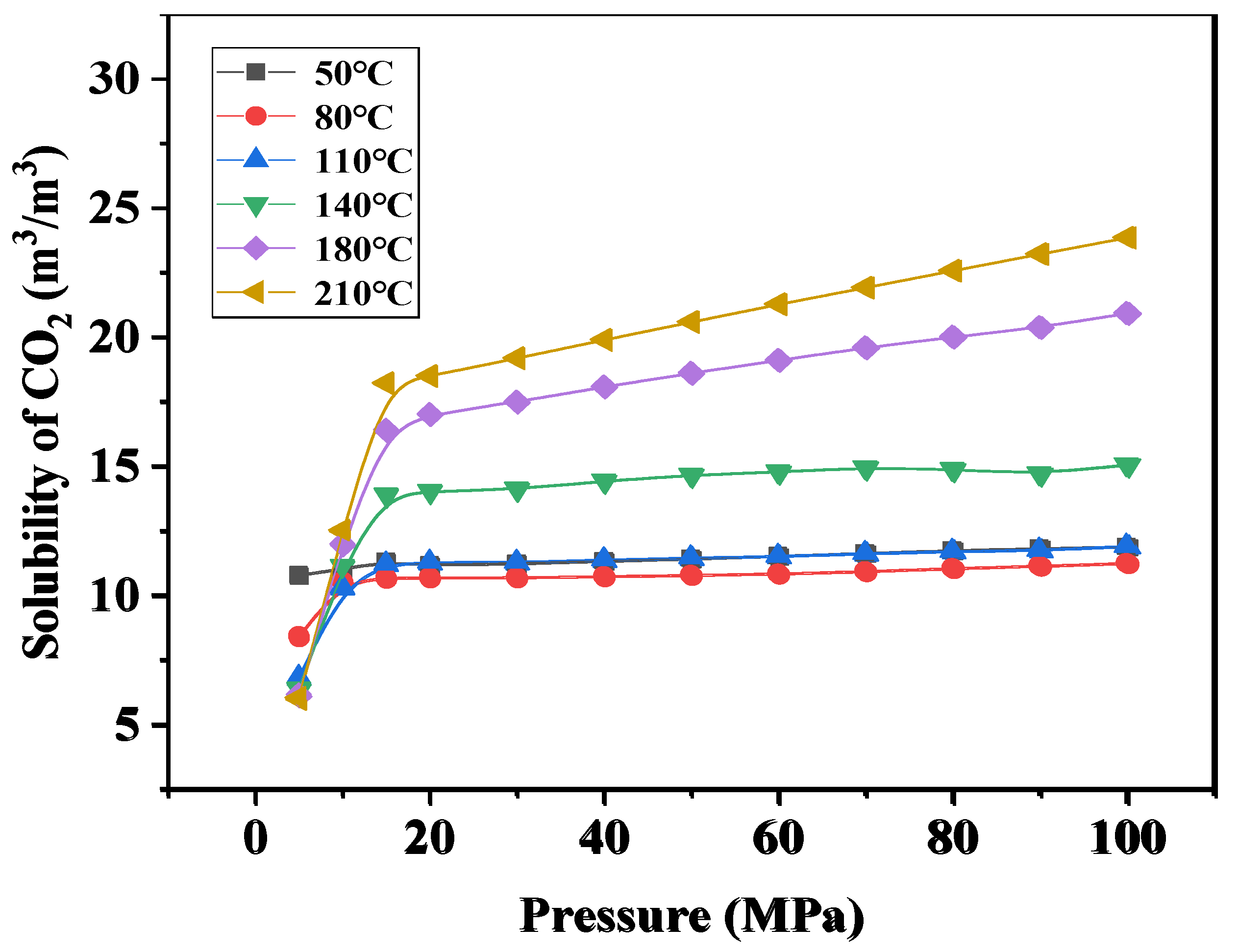
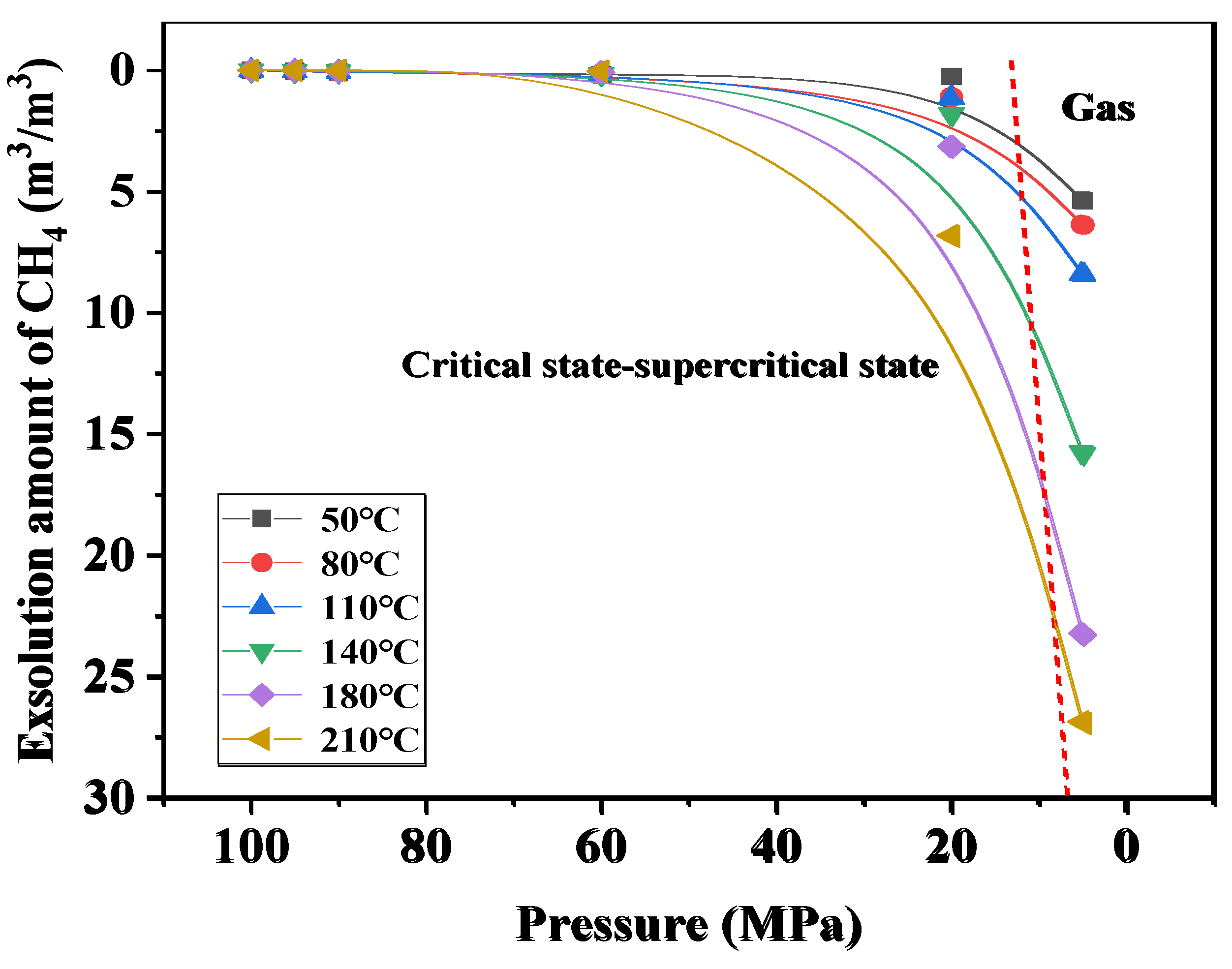
4.3. Study on the Exsolution Law of CO2 and CH4
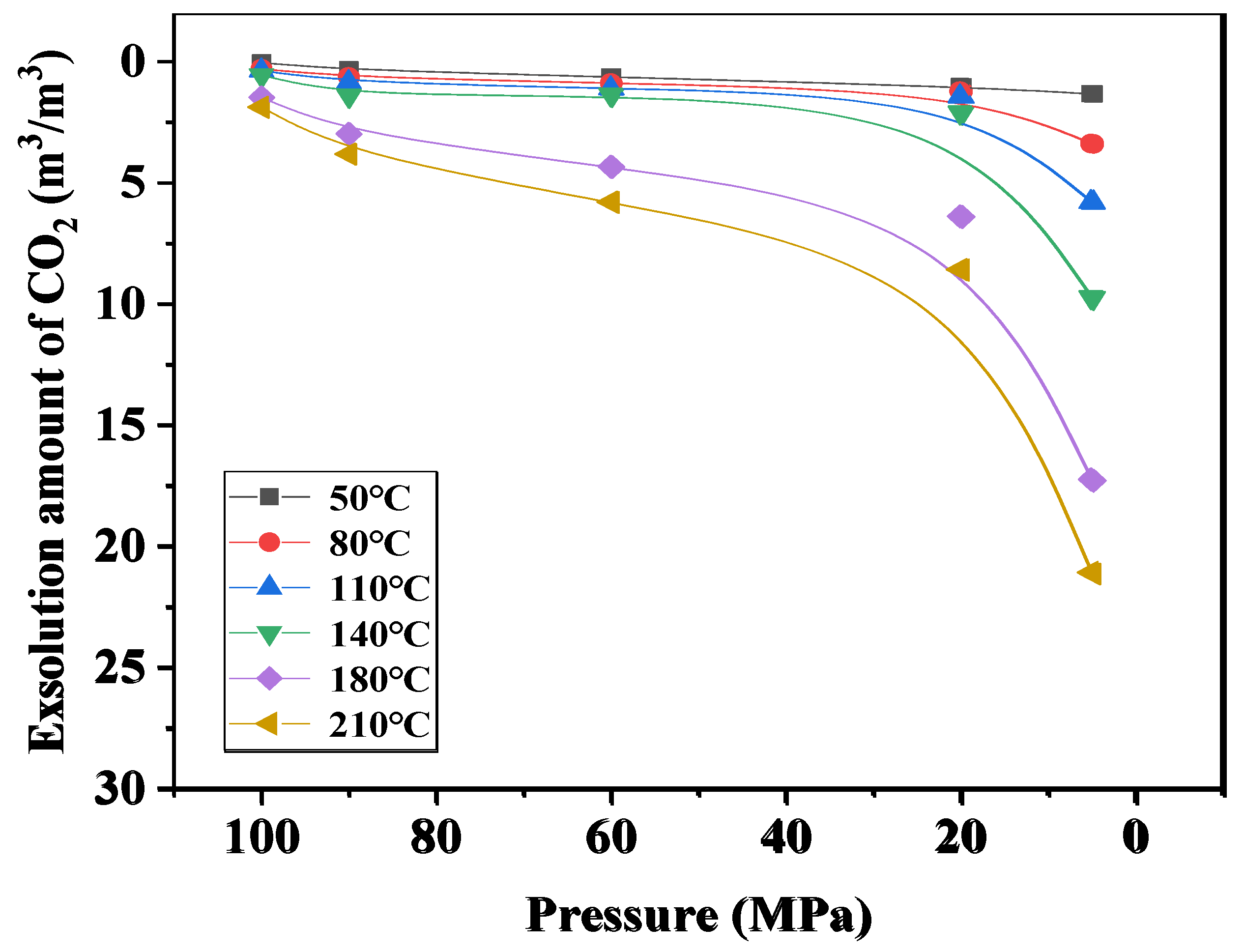
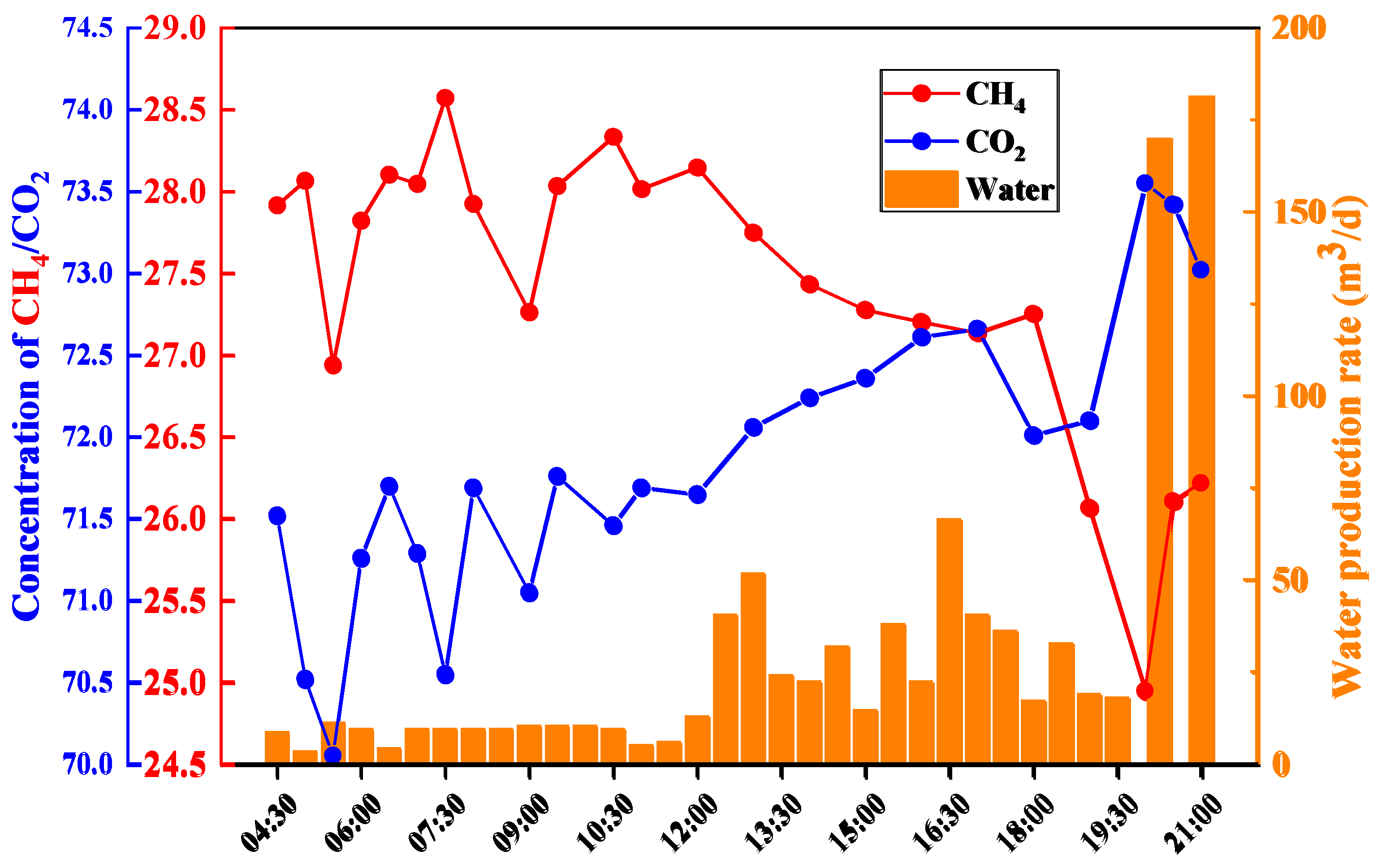
4.4. Application Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurnia K A, How C J, Matheswaran P ,et al. Insight into the molecular mechanism that controls the solubility of CH4 in ionic liquids. The Royal Society of Chemistry, 44(2020) 354-360. [CrossRef]
- Li R, Jiang P X, He D ,et al. Experimental investigation on the behavior of supercritical CO2 during reservoir depressurization. Environmental Science & Technology, 15(2017) 8869-8876. [CrossRef]
- Oldenburg, C. M, C. Doughty, and N. Spycher. The role of CO2 in CH4 exsolution from deep brine: implications for geologic carbon sequestration. Greenhouse Gases Science & Technology, 3(2013) 359-377. [CrossRef]
- Xu R, Li R, Ma J ,et al. Effect of mineral dissolution/precipitation and CO2 exsolution on CO2 transport in geological carbon storage. Accounts of Chemical Research, 50(2017) 2056-2066. [CrossRef]
- Gao G, Huang Z L, Huang B J,et al. The solution and exsolution characteristics of natural gas components in water at high temperature and pressure and their geological meaning. Petroleum Science, 9(2012) 25-30. [CrossRef]
- Huang X, Guo X, Zhou X ,et al. Effects of water invasion law on gas wells in high temperature and high pressure gas reservoir with a large accumulation of water-soluble gas. Journal of Natural Gas Science and Engineering, 62(2019) 68-78. [CrossRef]
- Xie, Weidong, M. Wang, and H. Wang. Adsorption characteristics of CH4 and CO2 in shale at high pressure and temperature. ACS omega, 6(2021) 18527-18536. [CrossRef]
- Zhou G Z, Duan X G, Chang J ,et al. Investigation of CH4/CO2 competitive adsorption-desorption mechanisms for enhanced shale gas production and carbon sequestration using nuclear magnetic resonance. Energy, 278(2023) 127964. [CrossRef]
- Gao T, Shen T T, Lin W S, et al. Experimental determination of CO2 solubility in liquid CH4/N2 mixtures at cryogenic temperatures. Industrial & Engineering Chemistry Research, 51(2012) 9403-9408. [CrossRef]
- Bruusgaard H, Juan G. Beltran, Servio P. Solubility measurements for the CH4+CO2+H2O system under hydrate–liquid–vapor equilibrium. Fluid Phase Equilibria, 296(2010) 106-109. [CrossRef]
- Liu G F, He L Y, Fan Z Q ,et al. Investigation of gas solubility and its effects on natural gas reserve and production in tight formations. Fuel, 295(2021) 120507. [CrossRef]
- Kapoor U, and Shah J K.. Monte carlo simulations of pure and mixed gas solubilities of CO2 and CH4 in nonideal ionic liquid-ionic liquid mixtures. Industrial & Engineering Chemistry Research, 58(2020) 22569-22578. [CrossRef]
- Zhao Y, Yu J, Shi H ,et al. Study of methane solubility calculation based on modified Henry's law and BP neural network. Processes, 12(2024) 1091. [CrossRef]
- Zirrahi M, Azin R, Hassanzadeh H ,et al. Mutual solubility of CH4, CO2, H2S, and their mixtures in brine under subsurface disposal conditions. Fluid Phase Equilibria, 324(2012) 80-93. [CrossRef]
- Li L, Ye J, Li M ,et al. Investigation of the law of natural gas phase behavior during the migration and reservoir formation. Geofluids, 2022(2022) 13. [CrossRef]
- Kvam O, Sarkisov L. Solubility prediction in mixed solvents: a combined molecular simulation and experimental approach. Fluid Phase Equilibria, 484(2019) 26-37. [CrossRef]
- Liu F, Han C, Yu L ,et al. Solubility of H2S-CH4 mixtures in calcium chloride solution: Insight from molecular dynamics simulations. Journal of Molecular Liquids, 407(2024) 11. [CrossRef]
- Osullivan T D, Smith N O, and Nagy B.. Solubility of natural gases in aqueous salt solution nitrogen in aqueous NaCl at high pressures. Geochimica Et Cosmichimica Acta, 30(1966) 617. [CrossRef]
- Duffy J R, Smith N O, and Nagy B.. Solubility of natural gases in aqueous salt solutions.1. liquids surfaces in the system CH4-H2O-NaCl2-CaCl2 at room temperatures and at pressures below 1000 Psia. Geochimica Et Cosmichimica Acta, 24(1961) 23-31, https://doi.10.1016/0016-7037(61)90004-7.
- Smith N O, Kelemen S, and Nagy B.. Solubility of natural gases in aqueous salt solutions.2. nitrogen in aqueous NaCl, CaCl2, Na2SO4 and MgSO4 at room temperatures and at pressures below 1000 Psia. Geochimica Et Cosmichimica Acta, 26(1962) 921-926, https://doi.10.1016/0016-7037(62)90066-2.
- Yang K, Zhou J P, Xian X F ,et al. Gas adsorption characteristics changes in shale after supercritical CO2-water exposure at different pressures and temperatures. Fuel, 310(2022) 122260, https://doi.10.1016/j.fuel.2021.122260.
- Ma J, and Huang Z .. Experiments of methane gas solubility in formation water under high temperature and high pressure and their geological significance. Australian Journal of Earth Sciences, 64(2017) 335-342, https://doi.10.1080/08120099.2017.1294108.
- Gao J, Zheng D Q, Guo T M. Solubilities of methane, nitrogen, carbon dioxide, and a natural gas mixture in aqueous sodium bicarbonate solutions under high pressure and elevated temperature. Journal of Chemical & Engineering Data, 42(1997) 69-73, https://doi.10.1021/je960275n.
- He H K, Sun B J, Sun X H ,et al. Prediction method for methane solubility in high-temperature and high-pressure aqueous solutions in ultra-deep drilling. Geoenergy Science and Engineering, 223(2023) 211522, https://doi.10.1016/j.geoen.2023.211522.
- Huang X, Guo X, Zhou X,et al. Effects of water invasion law on gas wells in high temperature and high pressure gas reservoir with a large accumulation of water-soluble gas. Journal of Natural Gas Science and Engineering, 62(2019) 68-78, https://doi.10.1016/j.jngse.2018.11.
- Nabipour N, Qasem S N, Salwana E ,et al. Evolving LSSVM and ELM models to predict solubility of non-hydrocarbon gases in aqueous electrolyte systems. Measurement, 164(2020) 107999, https://doi.10.1016/j.measurement.2020.107999.
- Neisani Samani N, Miforughy S M, Safari H,et al. Solubility of hydrocarbon and non-hydrocarbon gases in aqueous electrolyte solutions: A reliable computational strategy. Fuel, 241(2019) 1026-1035, https://doi.10.1016/j.fuel.2018.11.
- Nakhaei-Kohani R, Taslimi-Renani E, Hadavimoghaddam F ,et al. Modeling solubility of CO2-N2 gas mixtures in aqueous electrolyte systems using artificial intelligence techniques and equations of state. Scientific Reports, 12(2022) 3625, https://doi.10.1038/s41598-022-07393-z.
- Zoghi A T, Feyzi F, Zarrinpashneh S,et al. Solubility of light reservoir gasses in water by the modified Peng-Robinson plus association equation of state using experimental critical properties for pure water. Journal of Petroleum Science and Engineering, 78(2011) 109-118, https://doi.10.1016/j.petrol.2011.05.
- Hemmati-Sarapardeh A, Amar M N, Soltanian M R,et al. Modeling CO2 solubility in water at high pressure and temperature conditions. Energy & Fuels, 34(2020) 4761-4776, https://doi.10.1021/acs.energyfuels.0c00114.
- Dhima A, De Hemptinne J C, Jose J. Solubility of hydrocarbons and CO2 mixtures in water under high pressure. Industrial & Engineering Chemistry Research, 38(1999) 3144-3161, https://doi.10.1021/IE980768G.
- Xie Y H, Huang B J. Characteristics and accumulation mechanisms of the Dongfang 13-1 high temperature and overpressured gas field in the Yinggehai Basin, the South China Sea. Science China-Earth Sciences, 57(2014) 2799-2807, https://doi.10.1007/s11430-014-4934-0.
- Taggart I. Extraction of dissolved methane in brines by CO2 injection: implication for CO2 sequestration. Spe Reservoir Evaluation & Engineering, 13(2010) 791-804, https://doi.10.2118/124630-PA.
- Wu Y, Li P. The potential of coupled carbon storage and geothermal extraction in a CO2-enhanced geothermal system: a review. Geothermal Energy, 8(2020) 19, https://doi.10.1186/s40517-020-00173-w.
- Caiwei F, Jiangjun C, Jinglan L ,et al. Heterogeneity and influencing factors of marine gravity flow tight sandstone under abnormally high pressure: a case study from the miocene Huangliu formation reservoirs in LD10 area, Yinggehai Basin, South China Sea. Petroleum Exploration and Development, 48(2021) 1048-1062, https://doi.10.1016/S1876-3804(21)60090-3.
- Yu J F, Song R Y, Pei J X ,et al. CO2 fluid flow patterns near major deep faults: geochemical and 3D seismic data from the Ying-Qiong basin of the south China sea. Geofluids, 2022(2022) 9962343, https://doi.10.1155/2022/9962343.
- Liu R, Heinemann N, Liu J,et al. CO2 sequestration by mineral trapping in natural analogues in the Yinggehai basin, south China sea. Marine & Petroleum Geology, 104(2019) 190-199, https://doi.10.1016/j.marpetgeo.2019.03.
- Sun Y, Lin R Y, Pan Y ,et al. Experimental analysis and numerical simulation of the stability of geological storage of CO2: a case study of transforming a depleted gas reservoir into a carbon sink carrier. Acs Omega, 6(2021) 34832-34841, https://doi.10.1021/acsomega.1c05475.
- Zhang X F, Song X H, Li X Y ,et al. Supercritical CO2 injection-induced fracturing in longmaxi shales: a laboratory study. Energies, 18(2025) 855, https://doi.10.3390/en18040855.
- Wang J, Wang Z S, Wang Y B ,et al. CO2 replacing CH4 behaviors and supercritical conditions. Energy & Fuels, 34(2020) 4353-4365. https://doi.10.1021/acs.energyfuels.0c00169.
- Li L L. Solubility and exsolution study of CO2 and CH4. Doctoral dissertation, 2018.
- Han S J, Sang S X, Duan P P ,et al. The effect of the density difference between supercritical CO2 and supercritical CH4 on their adsorption capacities: an experimental study on anthracite in the Qinshui Basin. Petroleum Science, 19(2022) 1516-1526, https://doi.10.1016/j.petsci.2022.03.003.

| Ion Type | Na++K+ | Mg2+ | Ca2+ | Cl- | SO42- | HCO3- | Total Salinity |
| Ion Content (mg/L) |
4884 | 6 | 3 | 2177 | 121 | 7100 | 14848 |
| Well | Layer | Pressure (MPa) |
Temperature (K) |
CH4 | CO2 | Cl- (mg/L) | Solubility (m3/m3) | Proportion of solution gas (%) | Gas type |
| LD10-X-10 | H2Ⅳ | 87.079 | 468.42 | 27.05 | 70.98 | 5000 | 47.6 | 0.54 | free gas |
| LD10-X-12 | H2V | 93.985 | 488.35 | 53.01 | 42.93 | 5400 | 41.25 | 100 | solution gas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




