Submitted:
18 July 2025
Posted:
21 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. CYP1B1 (OMIM #601771)
2.2. FOXC1 (OMIM #601090)
2.3. PAX6 (OMIM #607108)
2.4. LTBP2 (OMIM #602091) and ADAMTSL4 (OMIM #610113)
2.5. CPAMD8 (OMIM #608841)
2.6. COL4A1 (OMIM #120130) and PXDN (OMIM #605158)
2.7. TEK (OMIM #600221)
2.8. SLC4A11 (OMIM #610206)
2.9. ADAMTS18 (OMIM #607512)
2.10. PITX2 (OMIM #601542) and PITX3 (OMIM #602669)
2.11. FOXE3 (OMIM #601094)
2.12. SOX11 (OMIM #600898)
2.13. GJA8 (OMIM #600897)
2.14. KERA (OMIM #603288)
2.15. CDH2 (OMIM #114020), KDM5C (OMIM #314690) and TFAP2A (OMIM #107580)
3. Management of Childhood Glaucoma and Anterior Segment Dysgenesis
4. Molecular Therapies Under Development
4.1. Gene-Based Therapies for Glaucoma
4.2. Gene-Based Therapies for Aniridia
4. Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Anterior segment dysgenesis |
| BMP | Bone morphogenetic protein |
| CHED | Congenital hereditary endothelial dystrophy |
| CRISPR/Cas9 | Clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9 |
| DSAEK | Descemet stripping automated endothelial keratoplasty |
| ECM | Extracellular matrix |
| FDA | US Food and Drug Administration |
| IOP | Intraocular pressure |
| JOAG | Juvenile open angle glaucoma |
| MEK/ERK | Mitogen-activated protein kinase-ERK kinase/extracellular signal related kinase |
| MMCAT | Microcornea, myopic chorioretinal atrophy and telecanthus |
| MMP | Matrix metalloproteinase |
| MO | Morpholino oligonucleotide |
| OMIM | Online Mendelian Inheritance in Man |
| PCG | Primary congenital glaucoma |
| POAG | Primary open angle glaucoma |
| PTC | Premature termination codon |
| rAAV | Recombinant adeno-associated virus |
| rAd | Recombinant adenovirus |
| sgRNA | Single guide RNA |
| siRNA | Small interfering RNA |
| RGC | Retinal ganglion cell |
| RPE | Retinal pigment epithelium |
| STAR | Study of Ataluren in Participants With Nonsense Mutation Aniridia |
References
- Shaham, O.; Menuchin, Y.; Farhy, C.; Ashery-Padan, R. Pax6: A Multi-Level Regulator of Ocular Development. Prog. Retin. Eye Res. 2012, 31, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Cvekl, A.; Tamm, E.R. Anterior Eye Development and Ocular Mesenchyme: New Insights from Mouse Models and Human Diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 374. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Stegmaier, J.; Kobitski, A.Y.; Schott, B.; Weger, B.D.; Margariti, D.; Cereceda Delgado, A.R.; Gourain, V.; Scherr, T.; Yang, L.; et al. Pax6 Organizes the Anterior Eye Segment by Guiding Two Distinct Neural Crest Waves. PLoS Genet. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, I.; Rezaie, T.; Jansson, I.; Schenkman, J.B.; Sarfarazi, M. Expression of Cytochrome P4501b1 (Cyp1b1) during Early Murine Development. Mol. Vis. 2004, 10, 629–636. [Google Scholar] [PubMed]
- Davis-Silberman, N.; Ashery-Padan, R. Iris Development in Vertebrates; Genetic and Molecular Considerations. Brain Res. 2008, 1192, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Miesfeld, J.B.; Brown, N.L. Eye Organogenesis: A Hierarchical View of Ocular Development. Curr. Top. Dev. Biol. 2019, 132, 351–393. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, K.; Sugiura, T.; San Juan, R.G. The Development of Human Trabecular Meshwork. Med. Electron Microsc. 1994, 27, 275–281. [Google Scholar] [CrossRef]
- Marchini, G.; Toscani, M.; Chemello, F. Pediatric Glaucoma: Current Perspectives. Pediatr. Health Med. Ther. 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Thau, A.; Lloyd, M.; Freedman, S.; Beck, A.; Grajewski, A.; Levin, A.V. New Classification System for Pediatric Glaucoma: Implications for Clinical Care and a Research Registry. Curr. Opin. Ophthalmol. 2018, 29, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Papadopoulos, M.; Khaw, P.T. Primary Congenital Glaucoma. Prog. Brain Res. 2015, 221, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Seese, S.; Costakos, D.; Semina, E.V. Congenital Anterior Segment Ocular Disorders: Genotype-Phenotype Correlations and Emerging Novel Mechanisms. Prog. Retin. Eye Res. 2024, 101288. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hayward, J.D.; Tailor, V.; Nyanhete, R.; Ahlfors, H.; Gabriel, C.; Jannini, T.B.; Abbou-Rayyah, Y.; Henderson, R.; Nischal, K.K.; et al. The Oculome Panel Test: Next-Generation Sequencing to Diagnose a Diverse Range of Genetic Developmental Eye Disorders. Ophthalmology 2019, 126, 888–907. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Semina, E.V. Genetics of Anterior Segment Dysgenesis Disorders. Curr. Opin. Ophthalmol. 2011, 22, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, M.; Hanson, I.; van Heyningen, V. Aniridia. Eur. J. Hum. Genet. 2012, 20, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Maheshwari, M.; Capasso, J.; Atilla, H.; Dudakova, L.; Thompson, S.; Zitano, L.; Lay-Son, G.; Lowry, R.B.; Black, J.; et al. Axenfeld-Rieger Syndrome: More than Meets the Eye. J. Med. Genet. 2023, 60, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.S.W.; Ruddle, J.B.; Taranath, D.A.; Goldberg, I.; Smith, J.E.H.; Gole, G.; Chiang, M.Y.; Willett, F.; D’Mellow, G.; Breen, J.; et al. Childhood and Early Onset Glaucoma Classification and Genetic Profile in a Large Australasian Disease Registry. Ophthalmology 2021, 128, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Wowra, B.; Dobrowolski, D.; Parekh, M.; Wylęgała, E. General Treatment and Ophthalmic Management of Peters’ Anomaly. J. Clin. Med. 2024, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Moosajee, M.; Hingorani, M.; Moore, A.T. PAX6-Related Aniridia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington, Seattle: Seattle (WA), 1993. [Google Scholar]
- Jackson, D.; Malka, S.; Harding, P.; Palma, J.; Dunbar, H.; Moosajee, M. Molecular Diagnostic Challenges for Non-retinal Developmental Eye Disorders in the United Kingdom. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Home - OMIM. Available online: https://omim.org/ (accessed on 1 October 2024).
- Vincent, A.; Billingsley, G.; Priston, M.; Glaser, T.; Oliver, E.; Walter, M.; Ritch, R.; Levin, A.; Heon, E. Further Support of the Role of CYP1B1 in Patients with Peters Anomaly. Mol. Vis. 2006, 12, 506–510. [Google Scholar] [PubMed]
- Edward, D.; Rajhi, A.A.; Lewis, R.A.; Curry, S.; Wang, Z.; Bejjani, B. Molecular Basis of Peters Anomaly in Saudi Arabia. Ophthalmic Genet. 2004, 25, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Churchill, A.J.; Yeung, A. A Compound Heterozygous Change Found in Peters’ Anomaly. Mol. Vis. 2005, 11, 66–70. [Google Scholar] [PubMed]
- Stingl, J.V.; Diederich, S.; Diel, H.; Schuster, A.K.; Wagner, F.M.; Chronopoulos, P.; Aghayeva, F.; Grehn, F.; Winter, J.; Schweiger, S.; et al. First Results from the Prospective German Registry for Childhood Glaucoma: Phenotype-Genotype Association. J. Clin. Med. 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, M.; Dada, T.; Dada, R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Rep. Med. 2010, 2010, 212656. [Google Scholar] [CrossRef] [PubMed]
- Samant, M.; Chauhan, B.K.; Lathrop, K.L.; Nischal, K.K. Congenital Aniridia: Etiology, Manifestations and Management. Expert Rev. Ophthalmol. 2016, 11, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Gagrani, M.; Scanga, H.L.; Areaux, R.G.; Chu, C.T.; Nischal, K.K. Variable Phenotype of Congenital Corneal Opacities in Biallelic CYP1B1 Pathogenic Variants. Cornea 2024, 43, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Saei, O.; Malka, S.; Owen, N.; Aliyev, E.; Vempalli, F.R.; Ocieczek, P.; Al-Khathlan, B.; Genomics England Research Consortium; Fakhro, K.; Moosajee, M. Increasing the Diagnostic Yield of Childhood Glaucoma Cases Recruited into the 100,000 Genomes Project. BMC Genomics 2024, 25, 484. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Yousoof, S.; Grigg, J.R.; Flaherty, M.; Minoche, A.E.; Cowley, M.J.; Nash, B.M.; Ho, G.; Gayagay, T.; Lai, T.; et al. Revealing Hidden Genetic Diagnoses in the Ocular Anterior Segment Disorders. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, S.; Hodapp, E.; Chang, T.C.; Swols, D.M.; Cengiz, F.B.; Guo, S.; Zafeer, M.F.; Seyhan, S.; Bademci, G.; Scott, W.K.; et al. Spectrum of Genetic Variants Associated with Anterior Segment Dysgenesis in South Florida. Genes 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Collantes, E.R.A.; Delfin, M.S.; Fan, B.; Torregosa, J.M.R.; Siguan-Bell, C.; de Guzman Florcruz, N.V.; Martinez, J.M.D.; Masna-Hidalgo, B.J.; Guzman, V.P.T.; Anotado-Flores, J.F.; et al. EFEMP1 Rare Variants Cause Familial Juvenile-Onset Open Angle Glaucoma. Hum. Mutat. 2022, 43, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Aghayeva, F.A.; Schuster, A.K.; Diel, H.; Chronopoulos, P.; Wagner, F.M.; Grehn, F.; Pirlich, N.; Schweiger, S.; Pfeiffer, N.; Hoffmann, E.M. Childhood Glaucoma Registry in Germany: Initial Database, Clinical Care and Research (Pilot Study). BMC Res. Notes 2022, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, T.; Gan, J.; Mao, Y.; Zhao, L.; Jiao, X.; Fan, M.; Wang, T.; Zhang, D.; Xu, M.; et al. Zonule-Associated Gene Variants in Isolated Ectopia Lentis and Glaucoma. J. Glaucoma 2023, 32, e80–e89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-X.; Jia, W.-N.; Sun, Y.; Chen, T.-H.; Zhao, Z.-N.; Lan, L.-N.; Liu, Y.; Song, L.-H.; Jiang, Y.-X. Biallelic ADAMTSL4 Variants in a Chinese Cohort of Congenital Ectopia Lentis: Implications for Genotype-Phenotype Relationships. Hum. Mutat. 2022, 43, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, H.S.; Khan, A.O.; Patel, N.; Alkuraya, H.; Hashem, M.; Abdulwahab, F.; Ibrahim, N.; Aldahmesh, M.A.; Alkuraya, F.S. Congenital Glaucoma and CYP1B1: An Old Story Revisited. Hum. Genet. 2019, 138, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Souzeau, E.; Taranath, D.A.; Dubowsky, A.; Chappell, A.; Zhou, T.; Javadiyan, S.; Nicholl, J.; Kearns, L.S.; Staffieri, S.E.; et al. Biallelic CPAMD8 Variants Are a Frequent Cause of Childhood and Juvenile Open-Angle Glaucoma. Ophthalmology 2020, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Fernández, J.-M.; Aroca-Aguilar, J.-D.; Corton, M.; Ramírez, A.-I.; Alexandre-Moreno, S.; García-Antón, M.-T.; Salazar, J.-J.; Ferre-Fernández, J.-J.; Atienzar-Aroca, R.; Villaverde, C.; et al. CPAMD8 Loss-of-Function Underlies Non-Dominant Congenital Glaucoma with Variable Anterior Segment Dysgenesis and Abnormal Extracellular Matrix. Hum. Genet. 2020, 139, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.Y.; Costain, G.; Cytrynbaum, C.; Weksberg, R.; Cohn, R.D.; Ali, A. Novel Heterozygous Variants in PXDN Cause Different Anterior Segment Dysgenesis Phenotypes in Monozygotic Twins. Ophthalmic Genet. 2021, 42, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Rudkin, A.; Parry, D.A.; Burdon, K.P.; McKibbin, M.; Logan, C.V.; Abdelhamed, Z.I.A.; Muecke, J.S.; Fernandez-Fuentes, N.; Laurie, K.J.; et al. Homozygous Mutations in PXDN Cause Congenital Cataract, Corneal Opacity, and Developmental Glaucoma. Am. J. Hum. Genet. 2011, 89, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Zazo-Seco, C.; Plaisancié, J.; Bitoun, P.; Corton, M.; Arteche, A.; Ayuso, C.; Schneider, A.; Zafeiropoulou, D.; Gilissen, C.; Roche, O.; et al. Novel PXDN Biallelic Variants in Patients with Microphthalmia and Anterior Segment Dysgenesis. J. Hum. Genet. 2020, 65, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Lao, R.; Ling-Fung Tang, P.; Wan, E.; Mayer, W.; Bardakjian, T.; Shaw, G.M.; Kwok, P.-Y.; Schneider, A.; Slavotinek, A. Novel Mutations in PXDN Cause Microphthalmia and Anterior Segment Dysgenesis. Eur. J. Hum. Genet. EJHG 2015, 23, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, K.; Naz, S.; Mushtaq, A.; Wohler, E.; Sobreira, N.; Ho, B.-M.; Chen, L.-J.; Chu, W.-K.; Bashir, R. Exome Sequencing Reveals SLC4A11 Variant Underlying Congenital Hereditary Endothelial Dystrophy (CHED2) Misdiagnosed as Congenital Glaucoma. Genes 2023, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Tao, Y.F.; Mao, Y.K.; Chen, Z.J.; Wang, Y.; Hong, Y.F.; Fan, N. A family with developmental glaucoma and microcornea due to novel ADAMTS18 gene mutations. Zhonghua Yan Ke Za Zhi Chin. J. Ophthalmol. 2024, 60, 78–83. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Wu, Q.; Wei, X. Unilateral Buphthalmos, Corneal Staphyloma and Corneal Fistula Caused by Pathogenic Variant in the PITX3 Gene: A Case Report. BMC Ophthalmol. 2022, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Verdin, H.; Sorokina, E.A.; Meire, F.; Casteels, I.; de Ravel, T.; Semina, E.V.; De Baere, E. Novel and Recurrent PITX3 Mutations in Belgian Families with Autosomal Dominant Congenital Cataract and Anterior Segment Dysgenesis Have Similar Phenotypic and Functional Characteristics. Orphanet J. Rare Dis. 2014, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Vasanth, S.; Kabir, F.; Gottsch, J.D.; Khan, A.O.; Chaerkady, R.; Lee, M.-C.W.; Leitch, C.C.; Ma, Z.; Laux, J.; et al. FOXE3 Contributes to Peters Anomaly through Transcriptional Regulation of an Autophagy-Associated Protein Termed DNAJB1. Nat. Commun. 2016, 7, 10953. [Google Scholar] [CrossRef] [PubMed]
- Plaisancié, J.; Ragge, N.K.; Dollfus, H.; Kaplan, J.; Lehalle, D.; Francannet, C.; Morin, G.; Colineaux, H.; Calvas, P.; Chassaing, N. FOXE3 Mutations: Genotype-Phenotype Correlations. Clin. Genet. 2018, 93, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ormestad, M.; Blixt, A.; Churchill, A.; Martinsson, T.; Enerbäck, S.; Carlsson, P. Foxe3 Haploinsufficiency in Mice: A Model for Peters’ Anomaly. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1350–1357. [Google Scholar] [PubMed]
- Islam, L.; Kelberman, D.; Williamson, L.; Lewis, N.; Glindzicz, M.B.; Nischal, K.K.; Sowden, J.C. Functional Analysis of FOXE3 Mutations Causing Dominant and Recessive Ocular Anterior Segment Disease. Hum. Mutat. 2015, 36, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Qasim, M.; Ishaq, R.; Bukhari, S.A.; Sajid, Z.; Ashfaq, U.A.; Haque, A.; Ahmed, Z.M. Pathogenic Variants of AIPL1, MERTK, GUCY2D, and FOXE3 in Pakistani Families with Clinically Heterogeneous Eye Diseases. PLoS ONE 2020, 15, e0239748. [Google Scholar] [CrossRef] [PubMed]
- Sibon, I.; Coupry, I.; Menegon, P.; Bouchet, J.-P.; Gorry, P.; Burgelin, I.; Calvas, P.; Orignac, I.; Dousset, V.; Lacombe, D.; et al. COL4A1 Mutation in Axenfeld-Rieger Anomaly with Leukoencephalopathy and Stroke. Ann. Neurol. 2007, 62, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Coupry, I.; Sibon, I.; Mortemousque, B.; Rouanet, F.; Mine, M.; Goizet, C. Ophthalmological Features Associated with COL4A1 Mutations. Arch. Ophthalmol. Chic. Ill 1960 2010, 128, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Diel, H.; Ding, C.; Grehn, F.; Chronopoulos, P.; Bartsch, O.; Hoffmann, E.M. First Observation of Secondary Childhood Glaucoma in Coffin-Siris Syndrome: A Case Report and Literature Review. BMC Ophthalmol. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Houssin, N.S.; Zamora, C.; Abdul-Rahman, O.; Kalish, J.M.; Zackai, E.H.; Plageman, T.F.; Semina, E.V. Novel Variants in CDH2 Are Associated with a New Syndrome Including Peters Anomaly. Clin. Genet. 2020, 97, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Atilla, H.; Kannu, P.; Schneider, A.; Thompson, S.; Bardakjian, T.; Semina, E.V. Distinct Roles of Histone Lysine Demethylases and Methyltransferases in Developmental Eye Disease. Genes 2023, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Weh, E.; Reis, L.M.; Happ, H.C.; Levin, A.V.; Wheeler, P.G.; David, K.L.; Carney, E.; Angle, B.; Hauser, N.; Semina, E.V. Whole Exome Sequence Analysis of Peters Anomaly. Hum. Genet. 2014, 133, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Iwata, T. Exploring the Genetic Landscape of Childhood Glaucoma. Child. Basel Switz. 2024, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Chakraborty, S.; Chakraborty, A.; Chakrabarti, S.; Ray, K. Functional and Structural Analyses of CYP1B1 Variants Linked to Congenital and Adult-Onset Glaucoma to Investigate the Molecular Basis of These Diseases. PloS One 2016, 11, e0156252. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, S.; Acharya, M.; Banerjee, D.; Bhattacharjee, A.; Ray, K. Molecular Basis for Involvement of CYP1B1 in MYOC Upregulation and Its Potential Implication in Glaucoma Pathogenesis. PloS One 2012, 7, e45077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Sorenson, C.M.; Teixeira, L.; Dubielzig, R.R.; Peters, D.M.; Conway, S.J.; Jefcoate, C.R.; Sheibani, N. Cyp1b1 Mediates Periostin Regulation of Trabecular Meshwork Development by Suppression of Oxidative Stress. Mol. Cell. Biol. 2013, 33, 4225–4240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, Y.; Oatts, J.T. Genetic Changes and Testing Associated with Childhood Glaucoma: A Systematic Review. PLOS ONE 2024, 19, e0298883. [Google Scholar] [CrossRef] [PubMed]
- López-Garrido, M.-P.; Medina-Trillo, C.; Morales-Fernandez, L.; Garcia-Feijoo, J.; Martínez-de-la-Casa, J.-M.; García-Antón, M.; Escribano, J. Null CYP1B1 Genotypes in Primary Congenital and Nondominant Juvenile Glaucoma. Ophthalmology 2013, 120, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Medina-Trillo, C.; Ferre-Fernández, J.-J.; Aroca-Aguilar, J.-D.; Bonet-Fernández, J.-M.; Escribano, J. Functional Characterization of Eight Rare Missense CYP1B1 Variants Involved in Congenital Glaucoma and Their Association with Null Genotypes. Acta Ophthalmol. (Copenh.) 2016, 94, e555–e560. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O. Genetics of Primary Glaucoma. Curr. Opin. Ophthalmol. 2011, 22, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Plásilová, M.; Stoilov, I.; Sarfarazi, M.; Kádasi, L.; Feráková, E.; Ferák, V. Identification of a Single Ancestral CYP1B1 Mutation in Slovak Gypsies (Roms) Affected with Primary Congenital Glaucoma. J. Med. Genet. 1999, 36, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Kidson, S.H.; Kume, T.; Deng, K.; Winfrey, V.; Hogan, B.L. The Forkhead/Winged-Helix Gene, Mf1, Is Necessary for the Normal Development of the Cornea and Formation of the Anterior Chamber in the Mouse Eye. Dev. Biol. 1999, 211, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Berry, F.B.; Lines, M.A.; Oas, J.M.; Footz, T.; Underhill, D.A.; Gage, P.J.; Walter, M.A. Functional Interactions between FOXC1 and PITX2 Underlie the Sensitivity to FOXC1 Gene Dose in Axenfeld-Rieger Syndrome and Anterior Segment Dysgenesis. Hum. Mol. Genet. 2006, 15, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Paylakhi, S.H.; Moazzeni, H.; Yazdani, S.; Rassouli, P.; Arefian, E.; Jaberi, E.; Arash, E.H.; Gilani, A.S.; Fan, J.-B.; April, C.; et al. FOXC1 in Human Trabecular Meshwork Cells Is Involved in Regulatory Pathway That Includes miR-204, MEIS2, and ITGβ1. Exp. Eye Res. 2013, 111, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Friedman, A.; Heaney, S.; Purcell, P.; Maas, R.L. Meis Homeoproteins Directly Regulate Pax6 during Vertebrate Lens Morphogenesis. Genes Dev. 2002, 16, 2097. [Google Scholar] [CrossRef] [PubMed]
- Medina-Trillo, C.; Sánchez-Sánchez, F.; Aroca-Aguilar, J.-D.; Ferre-Fernández, J.-J.; Morales, L.; Méndez-Hernández, C.-D.; Blanco-Kelly, F.; Ayuso, C.; García-Feijoo, J.; Escribano, J. Hypo- and Hypermorphic FOXC1 Mutations in Dominant Glaucoma: Transactivation and Phenotypic Variability. PLoS ONE 2015, 10, e0119272. [Google Scholar] [CrossRef] [PubMed]
- Harding, P.; Moosajee, M. The Molecular Basis of Human Anophthalmia and Microphthalmia. J. Dev. Biol. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.; Canto-Soler, M.V. Molecular Mechanisms of Optic Vesicle Development: Complexities, Ambiguities and Controversies. Dev. Biol. 2007, 305, 1. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Tibrewal, S.; Garg, A.; Vohra, M.; Ratna, R.; Sangwan, V.S. New Horizons in Aniridia Management: Clinical Insights and Therapeutic Advances. Taiwan J. Ophthalmol. 2023, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Schedl, A.; Ross, A.; Lee, M.; Engelkamp, D.; Rashbass, P.; van Heyningen, V.; Hastie, N.D. Influence of PAX6 Gene Dosage on Development: Overexpression Causes Severe Eye Abnormalities. Cell 1996, 86, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Bentley, A.J.; Martin, F.L.; Collinson, J.M.; Douvaras, P.; Hill, R.E.; Morley, S.D.; Fullwood, N.J.; West, J.D. Effects of Aberrant Pax6 Gene Dosage on Mouse Corneal Pathophysiology and Corneal Epithelial Homeostasis. PLoS ONE 2011, 6, e28895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Somarajan, B.I.; Gupta, S.; Mahalingam, K.; Singh, A.; Sharma, A. A New Association of PAX6 Variation with Juvenile Onset Open Angle Glaucoma. J. Hum. Genet. 2023, 68, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Haji-Seyed-Javadi, R.; Jelodari-Mamaghani, S.; Paylakhi, S.H.; Yazdani, S.; Nilforushan, N.; Fan, J.-B.; Klotzle, B.; Mahmoudi, M.J.; Ebrahimian, M.J.; Chelich, N.; et al. LTBP2 Mutations Cause Weill-Marchesani and Weill-Marchesani-like Syndrome and Affect Disruptions in the Extracellular Matrix. Hum. Mutat. 2012, 33, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Narooie-Nejad, M.; Paylakhi, S.H.; Shojaee, S.; Fazlali, Z.; Rezaei Kanavi, M.; Nilforushan, N.; Yazdani, S.; Babrzadeh, F.; Suri, F.; Ronaghi, M.; et al. Loss of Function Mutations in the Gene Encoding Latent Transforming Growth Factor Beta Binding Protein 2, LTBP2, Cause Primary Congenital Glaucoma. Hum. Mol. Genet. 2009, 18, 3969–3977. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, L.A.R.; Wang, L.W.; Bader, H.; Ho, J.C.; Majors, A.K.; Hollyfield, J.G.; Traboulsi, E.I.; Apte, S.S. ADAMTSL4, a Secreted Glycoprotein Widely Distributed in the Eye, Binds Fibrillin-1 Microfibrils and Accelerates Microfibril Biogenesis. Invest. Ophthalmol. Vis. Sci. 2012, 53, 461. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.-S.; Hentschel, L.; Davidson, A.E.; Gerrelli, D.; Davie, R.; Rizzo, R.; Pontikos, N.; Plagnol, V.; Moore, A.T.; Sowden, J.C.; et al. Mutations in CPAMD8 Cause a Unique Form of Autosomal-Recessive Anterior Segment Dysgenesis. Am. J. Hum. Genet. 2016, 99, 1338. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Tevar, A.; Bonet-Fernandez, J.-M.; Atienzar-Aroca, R.; Aroca-Aguilar, J.-D. Functional Interaction between Zebrafish Adamtsl4 and Cpamd8 Matrix Metalloproteinase-Related Genes: Implications in Ocular Anterior Segment Development and Glaucoma. Invest. Ophthalmol. Vis. Sci. 2024, 65, OD6. [Google Scholar]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in Normal Skin and in Pathological Processes. North Am. J. Med. Sci. 2012, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, H.-K.; Naidansuren, P.; Ham, K.A.; Choi, H.S.; Ahn, H.-Y.; Kim, M.; Kang, D.H.; Kang, S.W.; Joe, Y.A. Peroxidasin Is Essential for Endothelial Cell Survival and Growth Signaling by Sulfilimine Crosslink-Dependent Matrix Assembly. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10228–10241. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Dilworth, D.J.; Weng, Y.-C.; Gould, D.B. Developmental Distribution of Collagen IV Isoforms and Relevance to Ocular Diseases. Matrix Biol. J. Int. Soc. Matrix Biol. 2009, 28, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kizhatil, K.; Ryan, M.; Marchant, J.K.; Henrich, S.; John, S.W.M. Schlemm’s Canal Is a Unique Vessel with a Combination of Blood Vascular and Lymphatic Phenotypes That Forms by a Novel Developmental Process. PLoS Biol. 2014, 12, e1001912. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.R.; Souma, T.; Tompson, S.W.; Onay, T.; Kizhatil, K.; Siggs, O.M.; Feng, L.; Whisenhunt, K.N.; Yanovitch, T.L.; Kalaydjieva, L.; et al. Angiopoietin-1 Is Required for Schlemm’s Canal Development in Mice and Humans. J. Clin. Invest. 2017, 127, 4421–4436. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Parker, M.D. SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy. BioMed Res. Int. 2015, 2015, 475392. [Google Scholar] [CrossRef] [PubMed]
- Vilas, G.L.; Loganathan, S.K.; Liu, J.; Riau, A.K.; Young, J.D.; Mehta, J.S.; Vithana, E.N.; Casey, J.R. Transmembrane Water-Flux through SLC4A11: A Route Defective in Genetic Corneal Diseases. Hum. Mol. Genet. 2013, 22, 4579–4590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, M.; Zhang, Q.; Dang, S.; Zhang, W. ADAMTS18 Regulates Early Branching Morphogenesis of Lacrimal Gland and Has a Significant Association with the Risk of Dry Eye in Mice. Exp. Eye Res. 2022, 218, 109020. [Google Scholar] [CrossRef] [PubMed]
- Ataca, D.; Caikovski, M.; Piersigilli, A.; Moulin, A.; Benarafa, C.; Earp, S.E.; Guri, Y.; Kostic, C.; Arsenijevic, Y.; Soininen, R.; et al. Adamts18 Deletion Results in Distinct Developmental Defects and Provides a Model for Congenital Disorders of Lens, Lung, and Female Reproductive Tract Development. Biol. Open 2016, 5, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Aldahmesh, M.A.; Alshammari, M.J.; Khan, A.O.; Mohamed, J.Y.; Alhabib, F.A.; Alkuraya, F.S. The Syndrome of Microcornea, Myopic Chorioretinal Atrophy, and Telecanthus (MMCAT) Is Caused by Mutations in ADAMTS18. Hum. Mutat. 2013, 34, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Aldahmesh, M.A.; Khan, A.O.; Mohamed, J.Y.; Alkuraya, H.; Ahmed, H.; Bobis, S.; Al-Mesfer, S.; Alkuraya, F.S. Identification of ADAMTS18 as a Gene Mutated in Knobloch Syndrome. J. Med. Genet. 2011, 48, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Cvekl, A.; Camerino, M.J. Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology. Cells 2022, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Semina, E.V.; Ferrell, R.E.; Mintz-Hittner, H.A.; Bitoun, P.; Alward, W.L.M.; Reiter, R.S.; Funkhauser, C.; Daack-Hirsch, S.; Murray, J.C. A Novel Homeobox Gene PITX3 Is Mutated in Families with Autosomal-Dominant Cataracts and ASMD. Nat. Genet. 1998, 19, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Dodonova, S.O.; Zhu, F.; Dienemann, C.; Taipale, J.; Cramer, P. Nucleosome-Bound SOX2 and SOX11 Structures Elucidate Pioneer Factor Function. Nature 2020, 580, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Cizelsky, W.; Hempel, A.; Metzig, M.; Tao, S.; Hollemann, T.; Kühl, M.; Kühl, S.J. Sox4 And Sox11 Function during Xenopus Laevis Eye Development. PLOS ONE 2013, 8, e69372. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.R.; Wurm, A.; Sock, E.; Fuchshofer, R.; Wegner, M. The High–Mobility–Group Transcription Factor Sox11 Plays an Important Role During Anterior Eye Segment Development. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3874. [Google Scholar]
- Li, L.; Fan, D.-B.; Zhao, Y.-T.; Li, Y.; Yang, Z.-B.; Zheng, G.-Y. GJA8 Missense Mutation Disrupts Hemichannels and Induces Cell Apoptosis in Human Lens Epithelial Cells. Sci. Rep. 2019, 9, 19157. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.W.-Y.; Liu, C.-Y. Roles of Lumican and Keratocan on Corneal Transparency. Glycoconj. J. 2002, 19, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.H.; Conrad, G.W. The Keratocan Gene Is Expressed in Both Ocular and Non-Ocular Tissues during Early Chick Development. Matrix Biol. 2003, 22, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Gealy, E.C.; Kerr, B.C.; Young, R.D.; Tudor, D.; Hayes, A.J.; Hughes, C.E.; Caterson, B.; Quantock, A.J.; Ralphs, J.R. Differential Expression of the Keratan Sulphate Proteoglycan, Keratocan, during Chick Corneal Embryogenesis. Histochem. Cell Biol. 2007, 128, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Pellegata, N.S.; Dieguez-Lucena, J.L.; Joensuu, T.; Lau, S.; Montgomery, K.T.; Krahe, R.; Kivelä, T.; Kucherlapati, R.; Forsius, H.; de la Chapelle, A. Mutations in KERA, Encoding Keratocan, Cause Cornea Plana. Nat. Genet. 2000, 25, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Accogli, A.; Calabretta, S.; St-Onge, J.; Boudrahem-Addour, N.; Dionne-Laporte, A.; Joset, P.; Azzarello-Burri, S.; Rauch, A.; Krier, J.; Fieg, E.; et al. De Novo Pathogenic Variants in N-Cadherin Cause a Syndromic Neurodevelopmental Disorder with Corpus Callosum, Axon, Cardiac, Ocular, and Genital Defects. Am. J. Hum. Genet. 2019, 105, 854. [Google Scholar] [CrossRef] [PubMed]
- Karwacki-Neisius, V.; Jang, A.; Cukuroglu, E.; Tai, A.; Jiao, A.; Predes, D.; Yoon, J.; Brookes, E.; Chen, J.; Iberg, A.; et al. WNT Signalling Control by KDM5C during Development Affects Cognition. Nature 2024, 627, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Schorle, H.; Meier, P.; Buchert, M.; Jaenisch, R.; Mitchell, P.J. Transcription Factor AP-2 Essential for Cranial Closure and Craniofacial Development. Nature 1996, 381, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, Y.; Kwon, K.; Ismail, T.; Lee, H.-K.; Kim, C.; Park, J.-W.; Kwon, O.-S.; Kang, B.-S.; Lee, D.-S.; et al. Physiological Effects of KDM5C on Neural Crest Migration and Eye Formation during Vertebrate Development. Epigenetics Chromatin 2018, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Gestri, G.; Osborne, R.J.; Wyatt, A.W.; Gerrelli, D.; Gribble, S.; Stewart, H.; Fryer, A.; Bunyan, D.J.; Prescott, K.; Collin, J.R.O.; et al. Reduced TFAP2A Function Causes Variable Optic Fissure Closure and Retinal Defects and Sensitizes Eye Development to Mutations in Other Morphogenetic Regulators. Hum. Genet. 2009, 126, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Phan, A.-V.; Yu, W.; Guo, Y.; Thompson, J.; Coppinger, C.; Venugopalan, S.R.; Amendt, B.A.; Van Otterloo, E.; Cao, H. Transcriptional Programs of Pitx2 and Tfap2a/Tfap2b Controlling Lineage Specification of Mandibular Epithelium during Tooth Initiation. PLOS Genet. 2024, 20, e1011364. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Chakrabarti, D.; Gothwal, V.K. Approach to Primary Congenital Glaucoma: A Perspective. Taiwan J. Ophthalmol. 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Coviltir, V.; Marinescu, M.C.; Urse, B.M.; Burcel, M.G. Primary Congenital and Childhood Glaucoma—A Complex Clinical Picture and Surgical Management. Diagnostics 2025, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Ghaffari, R.; Mohebi, M. Posterior Lamellar Keratoplasty (DSAEK) in Peters Anomaly. Cornea 2012, 31, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Research, C. for B.E. and Approved Cellular and Gene Therapy Products. FDA 2024. [Google Scholar]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene Therapy in the Anterior Eye Segment. Curr. Gene Ther. 2022, 22, 104–131. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili Mohanna, S.Z.; Korecki, A.J.; Simpson, E.M. rAAV-PHP.B Escapes the Mouse Eye and Causes Lethality Whereas rAAV9 Can Transduce Aniridic Corneal Limbal Stem Cells without Lethality. Gene Ther. 2023, 30, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9–Based Treatment of Myocilin-Associated Glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.; Gordon, E.M.; Li, L.; Liu, P.X.; Chen, Z.; Peng, H.; La Bree, L.; Anderson, W.F.; Hall, F.L.; McDonnell, P.J. Retroviral Gene Therapy Vectors for Prevention of Excimer Laser-Induced Corneal Haze. Invest. Ophthalmol. Vis. Sci. 2002, 43, 968–977. [Google Scholar] [PubMed]
- Patil, S.V.; Kaipa, B.R.; Ranshing, S.; Sundaresan, Y.; Millar, J.C.; Nagarajan, B.; Kiehlbauch, C.; Zhang, Q.; Jain, A.; Searby, C.C.; et al. Lentiviral Mediated Delivery of CRISPR/Cas9 Reduces Intraocular Pressure in a Mouse Model of Myocilin Glaucoma. Sci. Rep. 2024, 14, 6958. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Sharma, A.; Rodier, J.T.; Klibanov, A.M.; Rieger, F.G.; Mohan, R.R. BMP7 Gene Transfer via Gold Nanoparticles into Stroma Inhibits Corneal Fibrosis In Vivo. PLoS ONE 2013, 8, e66434. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.-C.; Chang, S.-F.; Liu, C.-Y.; Kao, W.W.-Y.; Huang, C.H.; Liaw, J. Eye Drop Delivery of Nano-Polymeric Micelle Formulated Genes with Cornea-Specific Promoters. J. Gene Med. 2007, 9, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Bae, J.A.; Park, H.J.; Im, S.K.; Oh, H.J.; Lin, X.H.; Kim, M.Y.; Lee, J.H.; Lee, S.E.; Ahn, K.Y.; et al. Subconjunctival Gene Delivery of the Transcription Factor GA-Binding Protein Delays Corneal Neovascularization in a Mouse Model. Gene Ther. 2009, 16, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Blair-Parks, K.; Weston, B.C.; Dean, D.A. High-Level Gene Transfer to the Cornea Using Electroporation. J. Gene Med. 2002, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, P.; Romano, V.; Rosetta, P.; Legrottaglie, E.F.; Kubrak-Kisza, M.; Azzolini, C.; Vinciguerra, R. Iontophoresis-Assisted Corneal Collagen Cross-Linking with Epithelial Debridement: Preliminary Results. BioMed Res. Int. 2016, 2016, 3720517. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; O’Callaghan, J.; Campbell, M. Gene Therapy for Glaucoma: Targeting Key Mechanisms. Vision Res. 2024, 225, None. [Google Scholar] [CrossRef] [PubMed]
- Sulak, R.; Liu, X.; Smedowski, A. The Concept of Gene Therapy for Glaucoma: The Dream That Has Not Come True Yet. Neural Regen. Res. 2023, 19, 92–99. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Delaney, C.; O’Connor, M.; van Batenburg-Sherwood, J.; Schicht, M.; Lütjen-Drecoll, E.; Hudson, N.; Ni Dhubhghaill, S.; Humphries, P.; Stanley, C.; et al. Matrix Metalloproteinase-3 (MMP-3)–Mediated Gene Therapy for Glaucoma. Sci. Adv. 2023, 9, eadf6537. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bell, O.H.; Copland, D.A.; Young, A.; Pooley, J.R.; Maswood, R.; Evans, R.S.; Khaw, P.T.; Ali, R.R.; Dick, A.D.; et al. Gene Therapy for Glaucoma by Ciliary Body Aquaporin 1 Disruption Using CRISPR-Cas9. Mol. Ther. 2020, 28, 820. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Song, M.; Stamer, W.D.; Qiao, Y.; Chen, X.; Sun, X.; Lei, Y.; Chen, J. miR-21-5p: A Viable Therapeutic Strategy for Regulating Intraocular Pressure. Exp. Eye Res. 2020, 200, 108197. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Gryciuk, A.; Gajewska-Woźniak, O.; Kordecka, K.; Boguszewski, P.M.; Waleszczyk, W.; Skup, M. Neuroprotection of Retinal Ganglion Cells with AAV2-BDNF Pretreatment Restoring Normal TrkB Receptor Protein Levels in Glaucoma. Int. J. Mol. Sci. 2020, 21, 6262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tang, L.; Zeng, J.; Chen, B. Adeno-Associated Virus Mediated SOD Gene Therapy Protects the Retinal Ganglion Cells from Chronic Intraocular Pressure Elevation Induced Injury via Attenuating Oxidative Stress and Improving Mitochondrial Dysfunction in a Rat Model. Am. J. Transl. Res. 2016, 8, 799–810. [Google Scholar] [PubMed]
- Richardson, R.; Smart, M.; Tracey-White, D.; Webster, A.R.; Moosajee, M. Mechanism and Evidence of Nonsense Suppression Therapy for Genetic Eye Disorders. Exp. Eye Res. 2017, 155, 24–37. [Google Scholar] [CrossRef] [PubMed]
- “pax6” [GENE] - ClinVar - NCBI. Available online: https://www.ncbi.nlm.nih.gov/clinvar (accessed on 27 January 2025).
- Lima Cunha, D.; Sarkar, H.; Eintracht, J.; Harding, P.; Zhou, J.H.; Moosajee, M. Restoration of Functional PAX6 in Aniridia Patient iPSC-Derived Ocular Tissue Models Using Repurposed Nonsense Suppression Drugs. Mol. Ther. Nucleic Acids 2023, 33, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Gregory-Evans, C.Y.; Wang, X.; Wasan, K.M.; Zhao, J.; Metcalfe, A.L.; Gregory-Evans, K. Postnatal Manipulation of Pax6 Dosage Reverses Congenital Tissue Malformation Defects. J. Clin. Invest. 2014, 124, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gregory-Evans, K.; Wasan, K.M.; Sivak, O.; Shan, X.; Gregory-Evans, C.Y. Efficacy of Postnatal In Vivo Nonsense Suppression Therapy in a Pax6 Mouse Model of Aniridia. Mol. Ther. Nucleic Acids 2017, 7, 417–428. [Google Scholar] [CrossRef] [PubMed]
- PTC Therapeutics. A Phase 2, Multicenter, Randomized, Double-Masked, Placebo-Controlled Study of the Safety and Efficacy of Ataluren (PTC124) for the Treatment of Nonsense Mutation Aniridia; clinicaltrials.gov; 2022.
- Mirjalili Mohanna, S.Z.; Hickmott, J.W.; Lam, S.L.; Chiu, N.Y.; Lengyell, T.C.; Tam, B.M.; Moritz, O.L.; Simpson, E.M. Germline CRISPR/Cas9-Mediated Gene Editing Prevents Vision Loss in a Novel Mouse Model of Aniridia. Mol. Ther. Methods Clin. Dev. 2020, 17, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Korecki, A.J.; Cueva-Vargas, J.L.; Fornes, O.; Agostinone, J.; Farkas, R.A.; Hickmott, J.W.; Lam, S.L.; Mathelier, A.; Zhou, M.; Wasserman, W.W.; et al. Human MiniPromoters for Ocular-rAAV Expression in ON Bipolar, Cone, Corneal, Endothelial, Müller Glial, and PAX6 Cells. Gene Ther. 2021, 28, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Dorot, O.; Roux, L.N.; Zennaro, L.; Oved, K.; Bremond-Gignac, D.; Pichinuk, E.; Aberdam, D. The Antipsychotropic Drug Duloxetine Rescues PAX6 Haploinsufficiency of Mutant Limbal Stem Cells through Inhibition of the MEK/ERK Signaling Pathway. Ocul. Surf. 2022, 23, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Oved, K.; Zennaro, L.; Dorot, O.; Zerbib, J.; Frank, E.; Roux, L.N.; Bremond-Gignac, D.; Pichinuk, E.; Aberdam, D. Ritanserin, a Potent Serotonin 2A Receptor Antagonist, Represses MEK/ERK Signalling Pathway to Restore PAX6 Production and Function in Aniridia-like Cellular Model. Biochem. Biophys. Res. Commun. 2021, 582, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Moustardas, P.; Abbasi, M.; Javidjam, D.; Asamoah, C.S.; Schweitzer-Chaput, A.; Cisternino, S.; Bremond-Gignac, D.; Aberdam, D.; Lagali, N. Duloxetine Enhances PAX6 Expression and Suppresses Innate Immune Responses in Murine LPS-Induced Corneal Inflammation. Ocul. Surf. 2024, 34, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Amini, M.; Moustardas, P.; Gutsmiedl, Q.; Javidjam, D.; Suiwal, S.; Seitz, B.; Fries, F.N.; Dashti, A.; Rautavaara, Y.; et al. Effects of miR-204-5p Modulation on PAX6 Regulation and Corneal Inflammation. Sci. Rep. 2024, 14, 26436. [Google Scholar] [CrossRef] [PubMed]
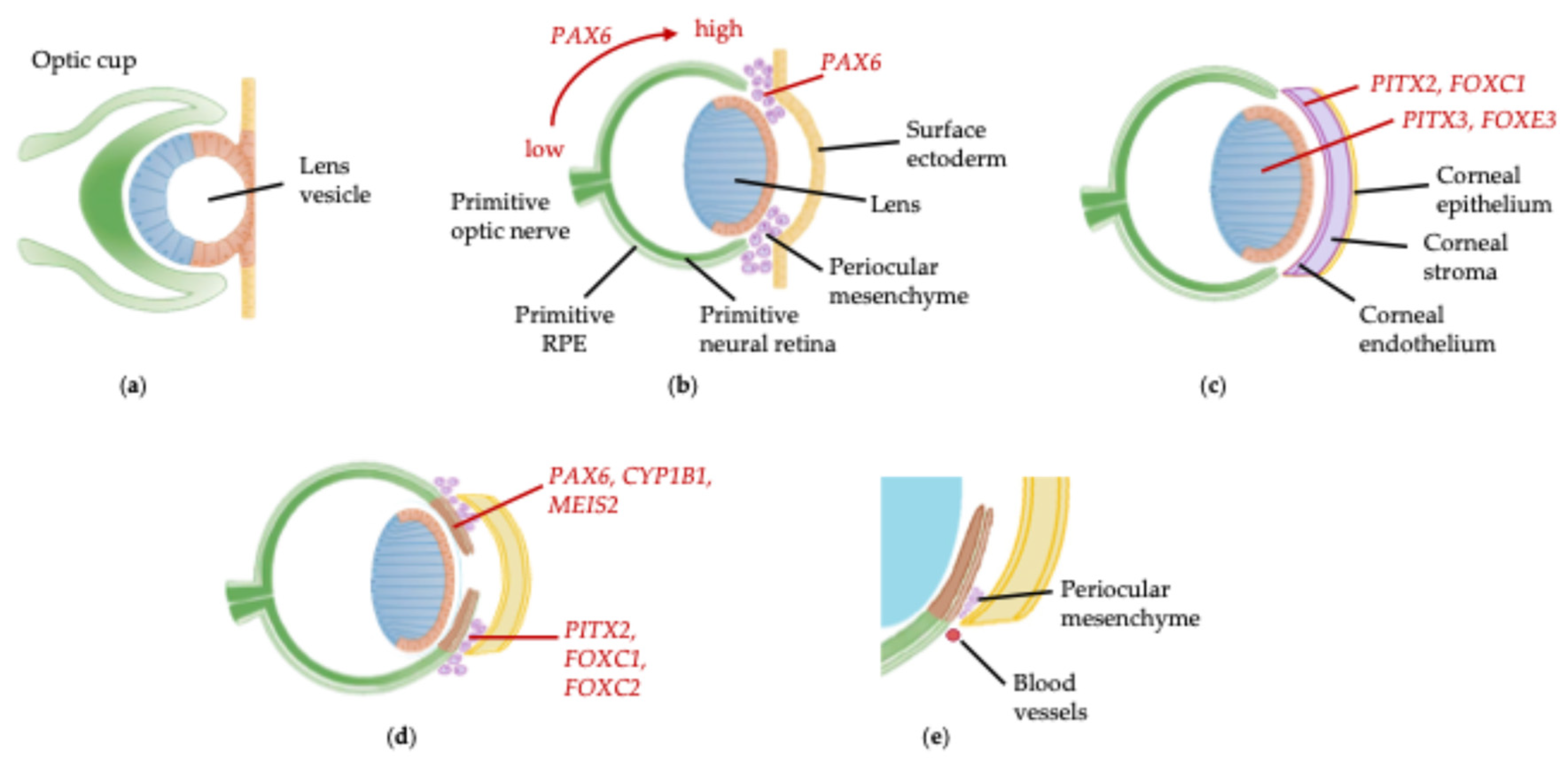

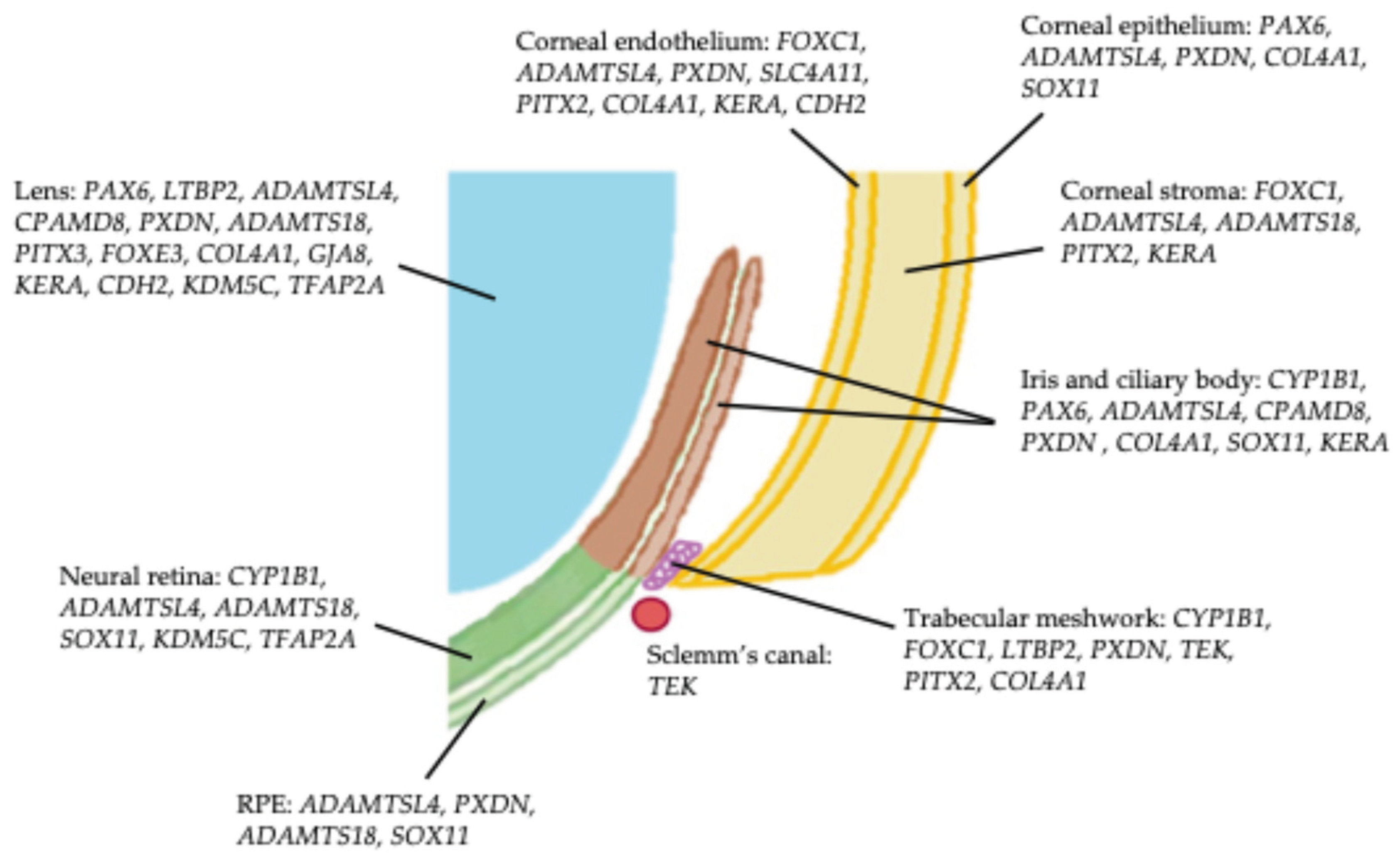
| Gene | OMIM ID | Phenotype(s) of anterior segment dysgenesis associated with childhood glaucoma | Associated with isolated childhood glaucoma |
|---|---|---|---|
| CYP1B1 | 601771 | Peters anomaly [20,21,22,23], Axenfeld-Rieger anomaly [24], aniridia [25], corneal dystrophy [12,26], unclassified ASD [27]. | Yes |
| FOXC1 | 601090 | Peters anomaly [13,28], Axenfeld-Rieger anomaly [15,23,29], aniridia [13], unclassified ASD [12,13]. | Yes |
| PAX6 | 607108 | Aniridia [16,29,30], Peters anomaly [28]. | Yes |
| LTBP2 | 602091 | Weill-Marchesani syndrome [23,31], congenital cataract [12], lenticular anomalies [16,32], unclassified ASD [16]. | Yes |
| ADAMTSL4 | 610113 | Ectopia lentis [33]. | Yes |
| CPAMD8 | 608841 | Lenticular anomalies [34,35], iris anomalies [35], unclassified ASD [16,36]. | Yes |
| PXDN | 605158 | Peters anomaly [37], congenital cataract [38], aphakia [37], aniridia [39], sclerocornea [28,40]. | Yes |
| TEK | 600221 | Sclerocornea [31]. | Yes |
| SLC4A11 | 610206 | Congenital hereditary endothelial dystrophy [16,27,41]. | Yes |
| ADAMTS18 | 607512 | Microcornea [42]. | Yes |
| PITX3 | 602669 | Microcornea [43,44], congenital cataract [44]. | Yes |
| PITX2 | 601542 | Peters anomaly [13,16], Axenfeld-Rieger anomaly [16,28], sclerocornea [28]. | No |
| FOXE3 | 601094 | Peters anomaly [45,46,47], iris anomalies [45,46], congenital cataract/aphakia [46,48], microphthalmia [46,49]. | No |
| COL4A1 | 120130 | Congenital cataract [13,50,51], Axenfeld-Rieger anomaly [51], iris anomalies [13,50]. | No |
| SOX11 | 600898 | Peters anomaly [52], aniridia [31,52]. | No |
| GJA8 | 600897 | Sclerocornea [23,28,31]. | No |
| KERA | 603288 | Unclassified ASD [34]. | No |
| CDH2 | 114020 | Peters anomaly [53]. | No |
| KDM5C | 314690 | Peters anomaly [54]. | No |
| TFAP2A | 107580 | Peters anomaly [55]. | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





