1. Introduction
Chondromyxoid fibroma (CMF) is among the rarest primary bone tumors, accounting for less than 2% of all benign and under 1% of all primary bone neoplasms. It was named based on its histological architecture, characterized by a mixture of cartilaginous, myxoid, and fibrous tissue components [
1,
2,
3,
4]. CMF was first described in 1948 as a distinct pathological entity by Dr. Henry L. Jaffe and Dr. Louis Lichtenstein in the journal
Archives of Pathology [
5]. A subsequent article by Dr. Lichtenstein in the
American Journal of Pathology further elaborated on the clinical, pathological, and radiological characteristics of CMF [
6]. At that time, differentiating this benign tumor from malignant chondrosarcoma posed a significant diagnostic challenge [
5,
6].
In a study of 36 cases published by Zillmer and Dorfman in 1989, misdiagnosis occurred in 22% of patients, with limb amputation mistakenly performed in two cases due to the erroneous diagnosis of chondrosarcoma [
2]. As of today, a PubMed search using the keyword "chondromyxoid fibroma" yields 612 articles, the majority of which are case reports (n = 370), case series, and 89 review articles. Although CMF remains diagnostically challenging, several well-established features are recognized. CMF most commonly arises in the metaphysis of long bones-especially the tibia and femur-typically affecting children and young adults during their second or third decades of life. It has a higher prevalence in males [
1,
5,
7,
8].
We present a rare case of CMF located in the distal femur of a 9-year-old girl and the diagnostic and clinical challenges observed four decades after the initial diagnosis and surgery. Notably, the patient is a practicing physician and one of the co-authors of this publication.
2. Case Presentation
This case was reconstructed based on the original medical documentation from 1985 and 1988, as well as heteroanamnesis from the patient's parents and anamnesis from the patient herself, who is now a physician.
In 1985, a previously healthy 9-year-old girl presented to the Division of Orthopedic Surgery, General Hospital Split, with limping and localized pain in the distal left femur. Radiographic evaluation revealed a large cystic lesion located in the metaphyseal-lateral aspect of the distal femur. At that time, advanced imaging modalities such as CT and MRI were not available in the hospital. A bone biopsy and curettage were performed in June 1985. Histopathological examination by two institutions-General Hospital Split and the Department of Pathology at the School of Medicine, University of Zagreb-confirmed the diagnosis of chondromyxoid fibroma. The term "fibromyxoid chondroma" was used in the report. The postoperative course was uneventful, and the patient returned to regular activities within a short period. Marginal resection and spongioplasty were discussed but not pursued.
Three years later, during routine follow-up, a small cortical lesion was identified on X-ray in the same metaphyseal region. Repeat curettage and biopsy were performed in June 1988. Postoperatively, the patient developed melena with a triple-positive benzidine test suggestive of gastrointestinal bleeding. Despite the unclear etiology, her condition stabilized, and she was discharged in good health. Histopathology once again confirmed the original diagnosis.
The patient underwent annual follow-up in subsequent years. Growth and development were normal, and there were no activity limitations. She pursued a medical career and is currently a specialist in internal medicine and gastroenterology, as well as a university assistant professor. She maintains an active lifestyle with regular engagement in cycling, swimming, brisk walking, yoga, and pilates.
At the age of 49, following stair climbing and repetitive squats, she experienced mild medial left knee pain, which worsened over two weeks. A physical medicine specialist noted mild swelling and tenderness at the pes anserinus region of the left limb and suspected overuse syndrome and bursitis. Treatment with Etoricoxib (90 mg/day), proton pump inhibitors, rest (RICE protocol), and physical therapy (magnetic and interferential currents) was initiated.
Given the prolonged interval since her last imaging evaluation, modern radiologic investigations were conducted. MRI and CT showed no abnormalities in the knee's soft tissues, but did reveal focal grade 2 patellar chondromalacia and minimal effusion in the suprapatellar recess. Importantly, four distinct lesions were identified in the left femoral diaphysis: three sharply demarcated round lesions (7-9 mm), and one subcortical lesion with sclerotic margins and imaging characteristics (low T1 signal, mixed T2 and PD signal with chondroid/fibrous components) consistent with residual CMF.
See Figure 1.
CT, axial image - sharply demarcated lesion with sclerotic border, thickened cortical bone, without agressive periostea reaction.

See Figure 2.
X ray - sharply demarcated lesion with sclerotic border, thickened cortical bone, without aggressive periosteal reaction.
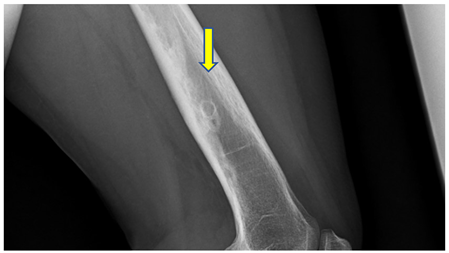
See Figure 3.
MRI: T2 weighted image, sagittal - sharply demarcated lesion with sclerotic border, high signal intensity in T2 i PD, low in T1, thickened cortical bone, without agressive periostea reaction
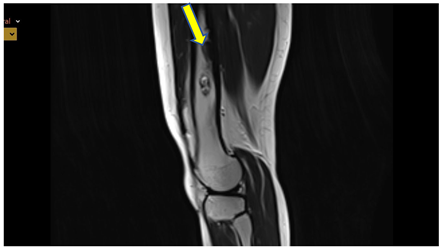
See Figure 4. and 5.
PD weighted image, axial and sagittal - sharply demarcated lesion with sclerotic border, high signal intensity in T2 i PD, low in T1, thickened cortical bone, without agressive periosteal reaction.
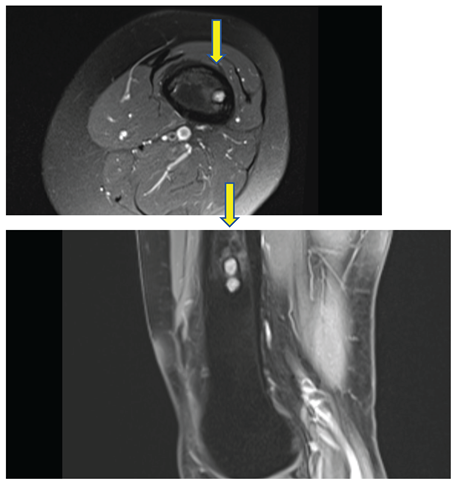
These lesions showed no signs of biological activity and were considered residual findings from childhood CMF. A follow-up MRI in three months was recommended to monitor potential changes.
To better localize the current pain, a diagnostic injection of corticosteroids and local anesthetics was applied to the pes anserinus area. The patient reported immediate relief, confirming the clinical diagnosis of bursitis. This case represents a highly instructive long-term example of chondromyxoid fibroma management, with both its classical and atypical elements. The initial surgical approach-intralesional curettage without adjunctive bone grafting-was consistent with the standard of care in the mid-1980s. Given the absence of CT or MRI at the time, histopathologic confirmation was crucial, and the dual-center verification added diagnostic robustness. The subsequent recurrence observed three years later reflects the well-documented biological behavior of CMF, which often includes local recurrence if intralesional excision is not combined with adjuvant measures such as high-speed burring, phenolization, or cryotherapy. Interestingly, in this case, the residual lesions identified four decades later remain radiologically inactive and clinically silent, further confirming the benign nature of CMF in the absence of additional triggers such as mechanical trauma or radiation. The patient's presentation with medial knee pain at age 49 was biomechanically unrelated to CMF residual lesions and instead attributable to pes anserinus bursitis-confirmed both clinically and therapeutically via targeted corticosteroid and anesthetic injection. However, her renewed imaging revealed small residual CMF-like lesions, highlighting the value of modern MRI/CT and PET/CT in distinguishing tumor residues from active disease. In light of current knowledge and imaging, no immediate orthopedic surgical intervention is warranted. Conservative observation with short-interval follow-up MRI is a sound strategy. Should any of the lesions demonstrate growth or signal characteristics suggesting aggressive behavior, percutaneous ablative techniques (cryoablation or radiofrequency ablation) could be reconsidered as minimally invasive alternatives to open surgery. This case illustrates the importance of long-term vigilance in CMF cases and confirms that not all residual lesions require surgical excision. Furthermore, it emphasizes the need for patient-centered, function-preserving decision-making, particularly in individuals with high physical activity levels and excellent quality of life.
3. Discussion
Chondromyxoid fibroma is a rare benign cartilaginous tumor, most commonly located in the metaphysis of long bones, with the proximal tibia being the most frequently affected site [
9]. Karaca et al. reported on 31 CMF cases over a 35-year period at their institution, identifying the pelvis (25.8%), proximal tibia (22.6%), and distal femur (12.9%) as the most prevalent localizations [
4]. Less frequent sites included the fibula, calcaneus, ribs, clavicle, olecranon, and bones of the hands and feet. Pain was the presenting symptom in 96.8% of patients, and swelling was reported in 41.9%. The recurrence rate was 16.1%, although no malignant transformation was observed. But, by our opinion it remains unclear which specific imaging modality was used to definitively distinguish true recurrence from residual post-surgical lesions in the reported cases. The study does not provide detailed information on whether postoperative imaging following the initial surgery confirmed complete clearance of the lesion. This raises an important methodological question: were the observed lesions during follow-up truly new recurrences, or could they have represent residues of the original tumor? Without early postoperative scans for comparison, it’s hard to draw a firm conclusion. A standardized radiological follow-up protocol, ideally including MRI or PET/CT, would help clarify this distinction in future studies.
It’s not entirely clear from the study which imaging methods were used to confirm that the lesions seen during follow-up were true recurrences rather than residual disease left behind after the first surgery. The authors don’t specify whether any postoperative imaging was performed to ensure that the bone was completely clear of tumor initially. This makes it difficult to say with certainty whether these follow-up findings truly represent new tumor growth or are simply longstanding remnants. Without early postoperative scans for comparison, it’s hard to draw a firm conclusion. Establishing a consistent follow-up imaging protocol — ideally including MRI or PET/CT — could help provide more clarity in similar cases in the future.
Standard treatment options for CMF include intralesional curettage, with or without bone grafting or cementing, wide resection, or segmental resection. The highest recurrence rates are associated with curettage performed without adjuvant techniques [
7,
8,
10,
11]. In the present case, intralesional curettage without grafting was performed, consistent with practices of the 1980s. A recurrence was observed three years later, requiring a second surgical intervention. Remarkably, the patient remained asymptomatic and functionally unrestricted for more than three decades thereafter. The reappearance of radiologically visible lesions in the same femoral region, identified through modern imaging nearly 40 years later, presents an important diagnostic dilemma: do these findings represent true late recurrences of CMF, or are they residual tumor remnants from the original disease that have remained biologically inactive over time?
This distinction is clinically significant. The lesions identified on MRI and CT were small, sharply demarcated, subcortical in location, and surrounded by sclerotic margins, without signs of cortical breakthrough or soft tissue invasion. Their imaging characteristics-low signal on T1 and mixed or intermediate signal on T2 and PD sequences-are consistent with CMF. Importantly, there was no peri-lesional edema or enhancement to suggest active inflammation or growth. Given the patient’s long symptom-free interval and excellent functional status, the current findings are most consistent with residual, inactive lesions rather than true recurrence. To further assess the metabolic activity of the lesions, a low density PET/CT scan was performed. The results showed no significant fluorodeoxyglucose (FDG) uptake in the femoral lesions, which strongly supports the interpretation that these are quiescent residuals rather than active or recurrent tumor tissue. Although CMF is typically a tumor of low metabolic activity, PET/CT can serve as a useful adjunct in ambiguous cases-especially when MRI findings are indeterminate or when clinical suspicion persists. The absence of FDG activity in this case provided additional reassurance and justified a conservative management approach with short-interval follow-up imaging. This case illustrates that not all post-treatment CMF lesions require excision, especially when they remain asymptomatic and radiologically stable. The patient’s current symptoms were unrelated to tumor recurrence and were instead due to pes anserinus bursitis—confirmed by clinical improvement following targeted corticosteroid injection. To our knowledge, only two cases of CMF have been reported in Croatia to date, both involving atypical locations-the mandible and the second metacarpal bone [
12,
13]. This case is the first Croatian report describing CMF of a long bone with long-term follow-up. It also underscores the importance of individualized, function-preserving decision-making and the value of a multidisciplinary approach. Contemporary management of CMF should involve orthopedic oncologists, radiologists, and, where appropriate, interventional radiologists, especially in the era of minimally invasive and image-guided techniques. While malignant transformation of CMF is exceedingly rare, it has been described. In their clinicopathologic review of 278 patients with chondromyxoid fibroma, Wu et al. provided valuable insights into the natural history and biological behavior of this rare tumor [
1], which is considered to have an extremely low risk of malignant transformation. Radiotherapy has been cited as a potential risk factor. Yadav et al. recently described a high-grade secondary chondrosarcoma developing in a previously treated CMF of the proximal tibia [
14], reinforcing the importance of careful monitoring over time. Conversely, Berenstein-Weyel et al. reported successful treatment of a pediatric CMF case using percutaneous radiofrequency ablation (RFA), and Gowda et al. described cryoablation in a case of recurrent CMF, both highlighting promising, less invasive therapeutic alternatives [
15,
16].
In summary, this case exemplifies the benign but persistent nature of CMF, the utility of modern imaging-including PET/CT-in guiding management decades after initial treatment, and the ongoing importance of long-term surveillance. It also raises critical questions about the interpretation of residual lesions and demonstrates how multimodal assessment can help resolve clinical uncertainty.
4. Conclusions
This case highlights several key points: knowledge of chondromyxoid fibroma has significantly improved over recent decades; advances in imaging modalities such as MRI and CT have facilitated earlier and more accurate diagnoses; lifelong clinical and radiologic surveillance is warranted due to the potential for late recurrence; malignant transformation, though rare, underscores the need for continued vigilance; a multidisciplinary approach involving orthopedic surgeons, radiologists, and interventional radiologists, is essential for optimal management.
Author Contributions
“Conceptualization, I.J. and J.V.; writing—original draft preparation, I.J.; writing—review and editing, J.V.; visualization, K.B.; supervision, A.B.
Funding
“This research received no external funding”-
Institutional Review Board Statement
This study protocol was reviewed and approved by the ethics committee of the Clinical Hospital Center in Split; class: 520-03/25-01/83; registry number: 2181-147-01-06/LJ.Z.-25-02, 02 April 2025. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Informed Consent Statement
Written informed consent was obtained from the patient.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
“The authors declare no conflicts of interest.”
Abbreviations
The following abbreviations are used in this manuscript:
| CMF |
chondromyxoid fibroma |
| MRI |
magnetic resonance imaging |
| CT |
computed tomography |
| PET-CT |
positron emission tomography and a computed tomography scan |
| RFA |
radiofrequency ablation |
| FDG |
fluorodeoxyglucose |
References
- Wu, C.; Inwards, C.Y.; O’Laughlin, S.; Rock, M.G.; Beabout, J.W.; Unni, K.K. Chondromyxoid fibroma of bone: A clinicopathologic review of 278 cases. Hum Pathol. [CrossRef]
- Zillmer, D.A.; Dorfman, H.D. Chondromyxoid fibroma of bone: Thirty-six cases with clinicopathologic correlation. Hum Pathol. [CrossRef]
- Campanacci, M. Chondromyxoid fibroma. In: Campanacci M, editor. Bone and Soft Tissue Sarcomas. New York: Springer; 1999. pp. 265–78.
- Karaca, M.O.; Özyıldıran, M.; Alizade, R.; Başarır, K.; Yıldız, H.Y. Chondromyxoid fibroma: A retrospective evaluation of 31 cases. Jt Dis Relat Surg. 3: 26;35(2). [CrossRef] [PubMed] [PubMed Central]
- Jaffe, H.L.; Lichtentsein, L. Chondromyxoid fibroma of bone; a distinctive benign tumor likely to be mistaken especially for chondrosarcoma. Arch Pathol (Chic). [PubMed]
- Lichtentsein, L. Chondromyxoid fibroma of bone. Am J Pathol. [PubMed]
- De Mattos, C.B.; Angsanuntsukh, C.; Arkader, A.; Dormans, J.P. Chondroblastoma and chondromyxoid fibroma. J Am Acad Orthop Surg. [CrossRef]
- Bhamra, J.S.; Al-Khateeb, H.; Dhinsa, B.S.; Gikas, P.D.; Tirabosco, R.; Pollock, R.C.; Skinner, J.A.; Aston, W.J.; Saifuddin, A.; Briggs, T.W. Chondromyxoid fibroma management: a single institution experience of 22 cases. World J Surg Oncol. 2: 12;12. [CrossRef] [PubMed] [PubMed Central]
- Soni, R.; Kapoor, C.; Shah, M.; Turakhiya, J.; Golwala, P. Chondromyxoid Fibroma: A Rare Case Report and Review of Literature. Cureus. 2016 Sep 23;8(9):e803. [CrossRef] [PubMed] [PubMed Central]
- Sharma, H.; Jane, M.J.; Reid, R. Chondromyxoid fibroma of the foot and ankle: 40 years' Scottish bone tumour registry experience. Int Orthop. [CrossRef]
- Lersundi, A.; Mankin, H.J.; Mourikis, A.; Hornicek, F.J. Chondromyxoid fibroma: A rarely encountered and puzzling tumor. Clin Orthop Relat Res. [CrossRef]
- Macan, D.; Cabov, T.; Uglesić, V. , Manojlović, S.; Kaleb, S.; Spicek, J. Chondromyxoid fibroma of the mandible. Br J Oral Maxillofac Surg. [CrossRef] [PubMed]
- Kirin, I.; Jurisić, D.; Mokrović, H.; Stanec, Z.; Stalekar, H. Chondromyxoid fibroma of the second metacarpal bone--a case report. Coll Antropol. [PubMed]
- Yadav, S.K.; Choudhary, A.K.; Kantiwal, P.; Karmakar, S.; Elhence, P.; Elhence, A. Chondrosarcoma proximal tibia secondary to chondromyxoid fibroma: A rare case report. Int J Surg Case Rep, 1108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berenstein-Weyel, T.; Lebel, E.; Katz, D.; Applbaum, Y.; Peyser, A. Chondromyxoid fibroma of the distal fibula treated by percutaneous radiofrequency ablation. J Orthop Surg (Hong Kong). 2309. [Google Scholar] [CrossRef] [PubMed]
- Gowda, P.C.; Dunlap, R.H.; Ahlawat, S.; Gross, J.M.; Morris, C.D.; Lyons, G.R. Recurrent chondromyxoid fibroma of the distal femur treated with percutaneous cryoablation. Skeletal Radiol. 2023; Erratum in: Skeletal Radiol. 2023 Dec;52(12):2503. 10.1007/s00256-023-04429-x. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








