Introduction
Pharmacological substances receive classification through the BCS framework based on their intestinal permeability combined with their water solubility characteristics [
1]. Intestinal permeability, dissolution rate, and solubility represent the three decisive elements which shape how rapidly and in what amount IR solid oral-dosage forms absorb medication through oral routes according to the BCS system. The criteria link to measured in vitro dissolution properties of drug products. The Biopharmaceutics Classification System (BCS) functions as a beneficial instrument for pharmaceutical development while also providing regulations for in vivo bioequivalence research exemptions. BCS provides a scientific explanation for describing the three key rate-limiting factors that affect oral absorption through its detailed descriptions.
(1) Drug release from the dose mechanisms
(2) The drug exists in dissolved form throughout the entire gastrointestinal fluid system. [
2]
(3) Drug compounds accessing the hepatic circulation through the GI membrane. [
3]
BCS class definitions start with the maximum strength found in IR products to determine drug solubility. A drug substance meets the highly soluble classification standard when its highest possible dosage strength completely dissolves in 250 milliliters or below of aqueous solution with a pH rating between 1.0 and 7.5. Otherwise, the classification is poor. The standard bioequivalence research approach that tests drugs on fasting volunteers using glass water amounts to 8 ounces gives rise to the 250 mL volume estimate. The permeability classification of medication substances is determined through measurements of human intestinal absorption rates or it derives from direct intestinal membrane permeability data. [
4]
If pharmacological drugs absorb 90 percent or more through the intestines, they are considered extremely permeable. According to this criterion, drug compounds having absorption levels less than 90% are categorized as inadequately permeable. Rapid dissolution is defined as the ability of a drug product to dissolve 85% or more of its labeled quantity in 30 minutes via USP Apparatus I at 100 rpm or USP Apparatus II at 50 rpm in 900 mL or fewer grams of the selected media, which includes acidic media with 0.1 N HCl or USP simulated intestinal fluid without enzymes, pH 4.5 buffer, or pH 6.8 buffer.
Through USP Apparatus I at 100 rpm or USP Apparatus II at 50 rpm in solutions no larger than 900 milliliter, the product quality control evaluates the drug ingredient for its dissolving time. Based on the medications' solubility and permeability in the GIT mucosa, the BCS divides them into four basic classes. These distinct divisions are found in each class of the biopharmaceutical categorization system.
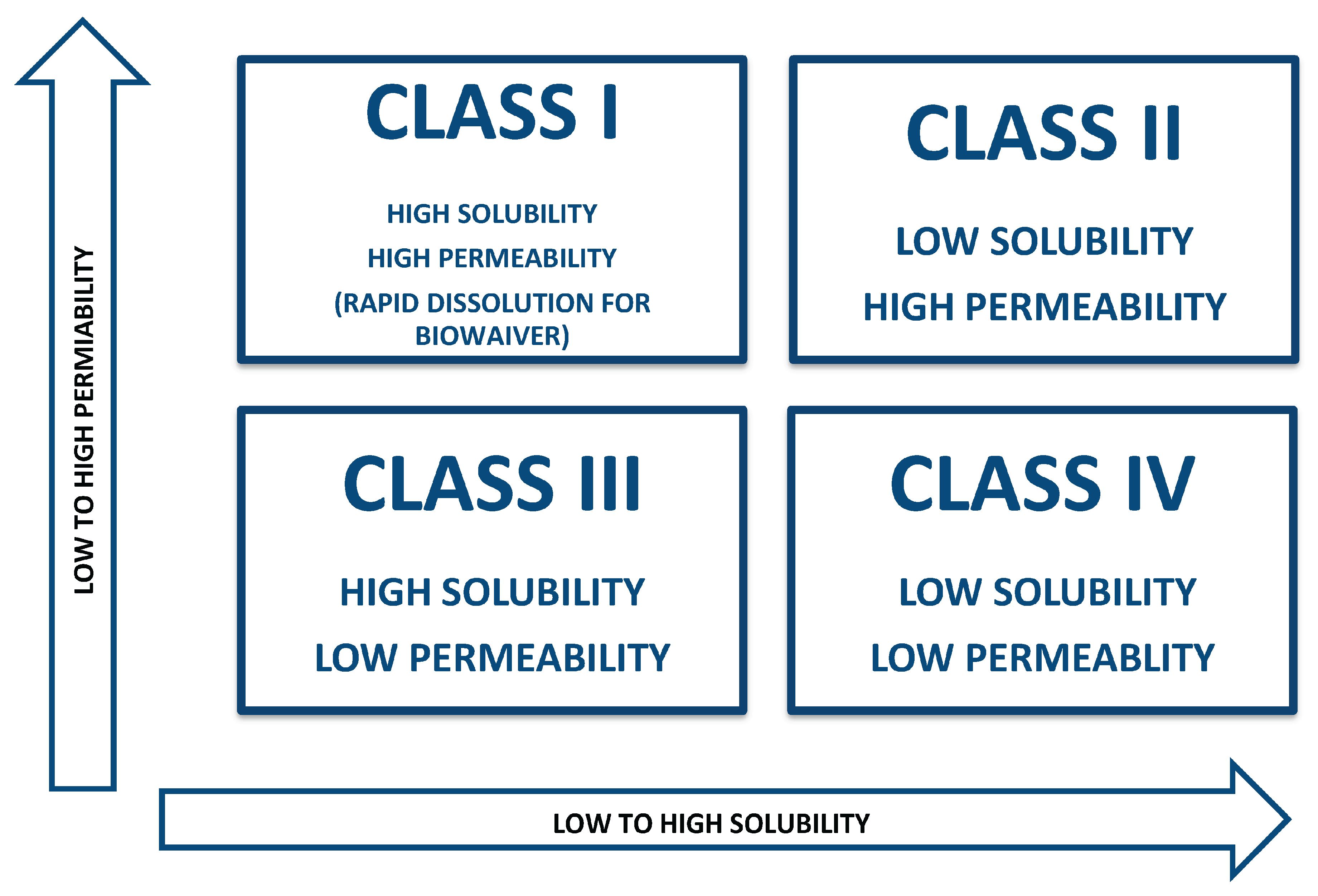
Class I: It is classification represents medications with strong permeability and solubility properties leading to maximum absorption in the body.
Class II: Medications having low solubility and strong permeability , absorb into body at a rate which depends on how fast they dissolve.
Class III: Low permeability, high solubility, and permeability-limited absorption
Class IV: Very low oral bioavailability because of low solubility and restricted permeability. [
5]
The Objectives
In vivo pharmaceutical efficiency evaluation relies on in vitro measurement data regarding permeability and solubility.
It is necessary to identify a class of oral immediate-release solid forms of medication since in vitro dissolving studies can verify their bioequivalence.
The paper presents systems which help classify pharmaceutical substances through analysis of their permeability characteristics combined with their solubility properties along with their dosage form dissolution properties. [
6]
The author proposes criteria to enable scientists to recognize disposable clinical bioequivalence tests that would maximize drug development and review process success.
The research presents classification methods which combine permeability and solubility characteristics of products with dosage form dissolution abilities. [
7]
Class of BCS Classification
Class I: The drug exhibit rapid absorption as well as dissolution inside the body similar to how oral dose work. Because Class I medicines rapidly absorb and dissolve within the body the measurements of bioequivalence together with bioavailability become irrelevant for these medications. The medications qualify as suitable controlled drug administration options when they demonstrate pharmacokinetic and pharmacodynamic suitability for the point. The drug molecules that release in these situations can be made of diverse substances such as constant surface area drug delivery shuttles, single composition osmotic tablet systems, microspheres, and multiporous oral drug absorption systems. The drug delivery approaches include matrix diffusion control systems and dual release drug absorption systems as well as granulated hydrogel systems and delayed pulsatile hydrogels together with stomach protective for absorption of drug and technology for Drug Delivery using pelletized pulsatile delivery systems and microparticles, as well as bioerodible improved medication absorption systems that are spheroidal, configurable, and comprise hydrogels for solubility control and the stabilizing mechanism of pellet delivery systems.
Class II: Drug dissolution rate determines bioavailability at this classification because molecules exhibit high permeability yet poor solubility. The pharmaceutical compounds display different bioavailability parameters which need multiple dissolving enhancement methods to boost their available quantities within the body. The medication design can incorporate controlled release functionality in products from this category. The list of pharmaceutical techniques includes solid dispersion methods together with emulsion or microemulsion systems and surfactants and lyophilized fast-melt systems and classical micronization and complexing agents such as cyclodextrins. Nanosized carriers such as nanoemulsion, nanosuspension, nanocrystals and Zer-Os tablet technology and Triglas, Soft Gel as well as the osmotic system have proven best in enhancing water-insoluble compounds' bioavailability by improving their solubility levels.
Class III: It is class of pharmacological substance requires intestinal membrane penetration to determine their absorption speed as their rate-limiting step. The bioavailability rate controls medication release from dosage forms because absorption limits based on how quickly permeation occurs. The ranitidine drugs with different dissolution properties produce the same plasma concentration-time profile when administered in vivo. The pharmaceutical ingredients possess low absorption potential so permeability enhancement becomes essential. Producing medications with controlled release features remains difficult for industries today.
Class IV: In this class, the drugs exhibit weak bioavailability together with variable responses. Intestinal permeability, stomach emptying, and drug dissolving rate are some of the variables that affect basic bioavailability. Oral administration is generally inappropriate for these medications but nano-suspensions and other drug delivery systems represent suitable options when medication use by mouth becomes necessary.
Figure 1.
The iilustration of BCS classification.
Figure 1.
The iilustration of BCS classification.
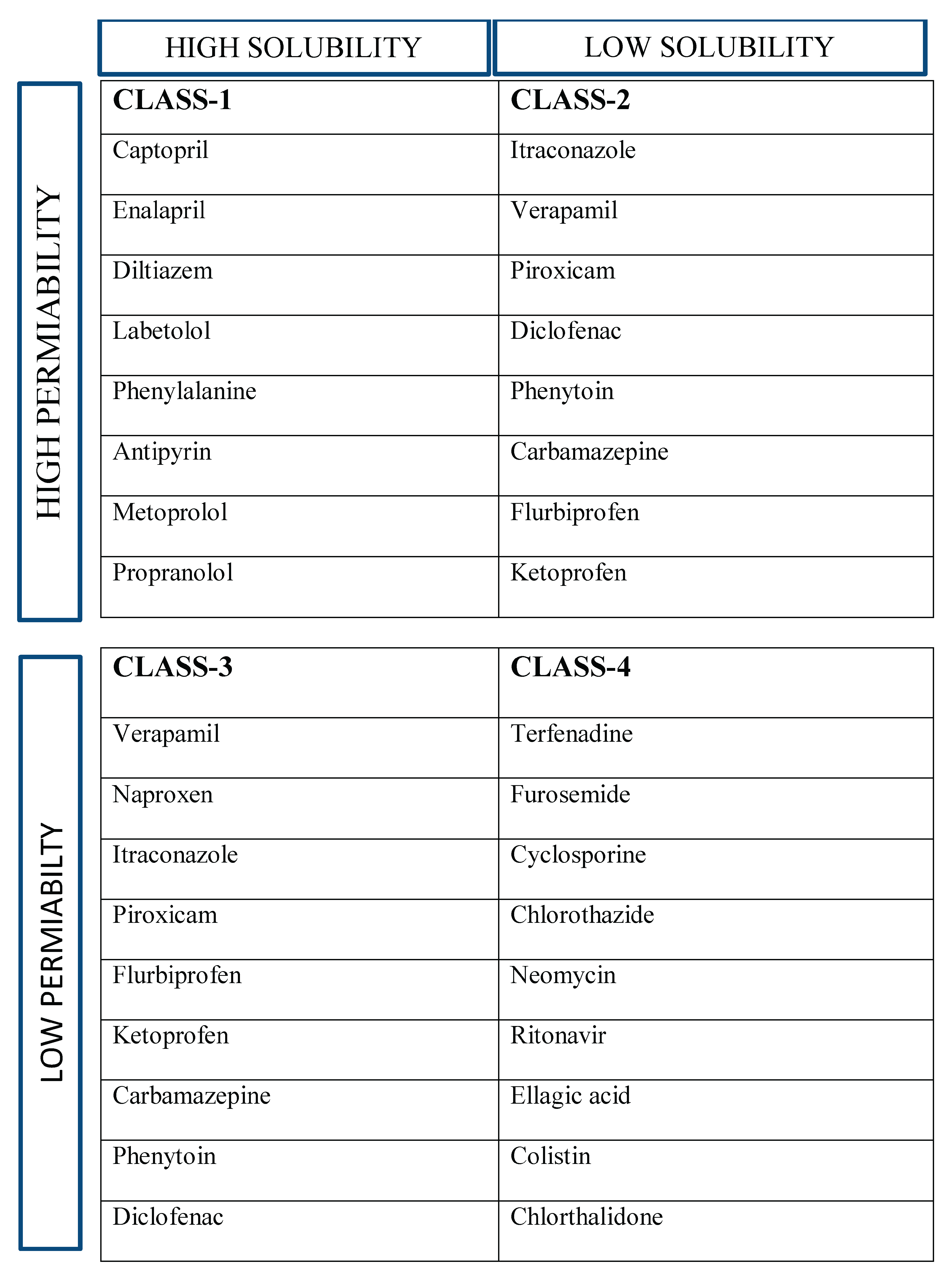
Table 1.
BCS CLASSIFICATION OF DRUG WITH EXAMPLES.
Table 1.
BCS CLASSIFICATION OF DRUG WITH EXAMPLES.
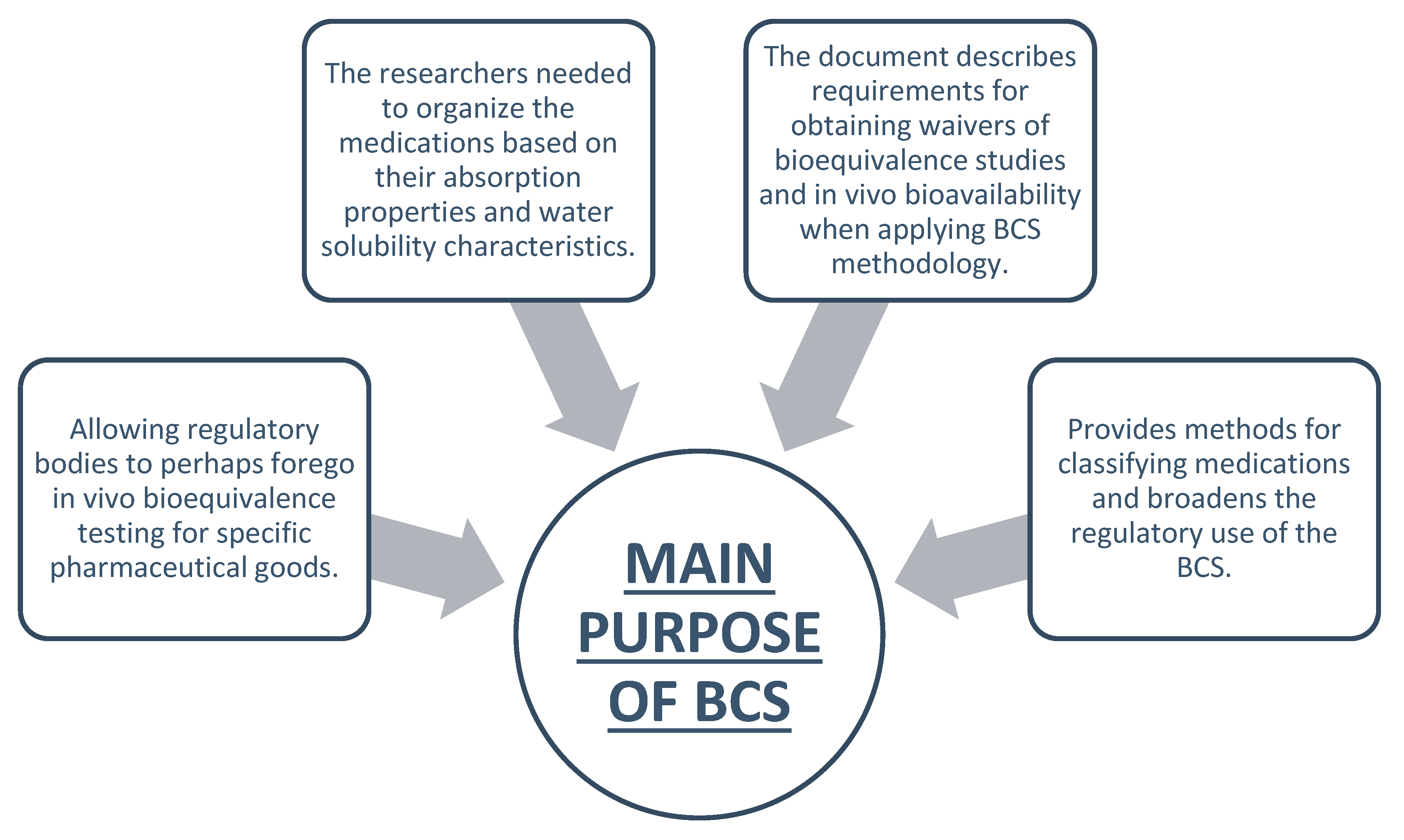
Main Purpose of BCS
Boundaries of BCS Class
The BCS classification can be determined through the border properties of solubility and permeability and dissolution.
Solubility: When a drug dissolves fully in 250 milliliters of water with a pH between 1 and 7.5 at 37 degrees Celsius, it is said to be very soluble.
Permeability: A pharmacological substance demonstrates high permeability when its absorption reaches or exceeds 90% of dose intake based on mass-balance methods or intravenous reference dosage comparison.
Dissolution: It takes place when drug substance ingredients reach 85% dissolution rate within 30-minute USP Apparatus 1 or 2 testing using 900 mL or less buffer solutions thus establishing the drug product as quickly dissolving. [
9]
An Essential Term Under BCS
Bioavaibility:
Bioavaibility represents the speed along with degree to which medications and their active elements enter bloodstream for delivery to the place of action.
Bioequivalence:
The same doses of two medication products become bioequivalent when their bioavailability exhibits similar absorption characteristics under similar testing environments. [
10]
Biopharmaceutics Drug Disposition Classification System (BDDCS):
The system described by Wu and Benet functions as an analytical tool that predicts the characteristics of drug disposition. The assessment system evaluation relies on data about solubility behavior alongside the rate of membrane permeation and the quantity of metabolic breakdown. This document reviews published BDDCS collection papers while assigning BDDCS classes to recently approved medications. Within its content the document presents documented sources used for parameter statements and it adjusts several previously recorded classification groups. This work examines how BDDCS classification benefits drug development projects before human or animal dosing of new molecular entities and reviews BCS class assignments versus BDDCS classifications when both systems exist. Furthermore it modifies the proposed solubility standards for prehuman-dose drug classification. [
11]
Solubility:
The GI tract absorption of new chemical entities remains restricted due to their low solubility rates which range between 40–70% of total compounds identified today. [
12] Research has identified key molecular features which determine poor aqueous hydration along with examination of strong crystal lattices preventing solubility. Compounds with stretched and rigid shapes that typically appear flat will remain soluble only when existing in the solid phase. These structural characteristics allow molecules to form tight crystals by engaging in H bonds, π–π stacking interactions as well as Van der Waals contacts resulting the strong intermolecular binding. The solvation-limited molecules, in contrast, are lipophilic, pliable, and bigger. [
13] Furthermore, certain intermediate compounds have a weak solvation and a strong crystal lattice. These molecules usually have a strong hydrogen bonding ability inside the crystal lattice and are tiny, stiff, and extremely lipophilic. Therefore, medicinal chemists can use the underlying molecular cause of low solubility to guide their techniques for improving water solubility. [
14]
Permiability
Absorption data obtained during human pharmacokinetic investigations using absolute efficiency and equilibrium balance techniques should be the preferable approach for assessing permeability. High permeability between the medication molecules and absorption vessels is indicated by a bioavailability rate of at least 85%. If the total percentage recovery of the parent medication plus Phase 1 oxidative metabolites and Phase 2 conjugative metabolites in urine surpasses 85% of the dosage that was delivered, high permeability may be deduced. Oxidative and conjugative metabolites serve as the entire basis for metabolite evaluation in feces. A scientific demonstration pointing to metabolite formation occurring after gastrointestinal absorption requires confirmation in order for reduction or hydrolysis metabolites to be excluded. The quantity of parent drug present in feces that stemmed from intestinal secretion or biliary excretion or resulted from unstable metabolite breakdown by microbial action should not be included in the absorption assessment. A pharmacological substance requires proof of high permeability to avoid BCS classification for low permeability. [
15]
Dissolution
It is the procedure in which a solid medicinal ingredient, such as that found in a tablet or capsule, decomposes and dissolves in a solvent, such as the fluids of your intestines or stomach, to create a solution. A drug's efficacy is ultimately determined by how quickly and how much of it is absorbed into the bloodstream, which is directly impacted by its rate and degree of breakdown. It is the process by which a solute dissolves into a solvent to form a solution combination. Using USP equipment 1 at 100 rpm or apparatus 2 at 50 rpm in 900 mL of buffer solutions (0.1 N HCl/pH 4.5 buffer/pH 6.8 buffer without enzymes), when 85% of the indicated amount of drug ingredient dissolved in 30 minutes, the drug product indicates to have rapid dissolution.[
16]
In vitro dissolution testing:. One popular technique for forecasting a medicinal product's in vivo (in the body) dissolve behaviour of drug product.
Studies on bioequivalence: To ascertain whether several formulations of the same medication dissolve and absorb similarly, dissolution profiles are utilized. [
17]
Comparable in vitro dissolving properties (f2 comparison) in each of the specified circumstances. Test product from a minimum 100,000 units, or 1/10 of the production size. [
18].
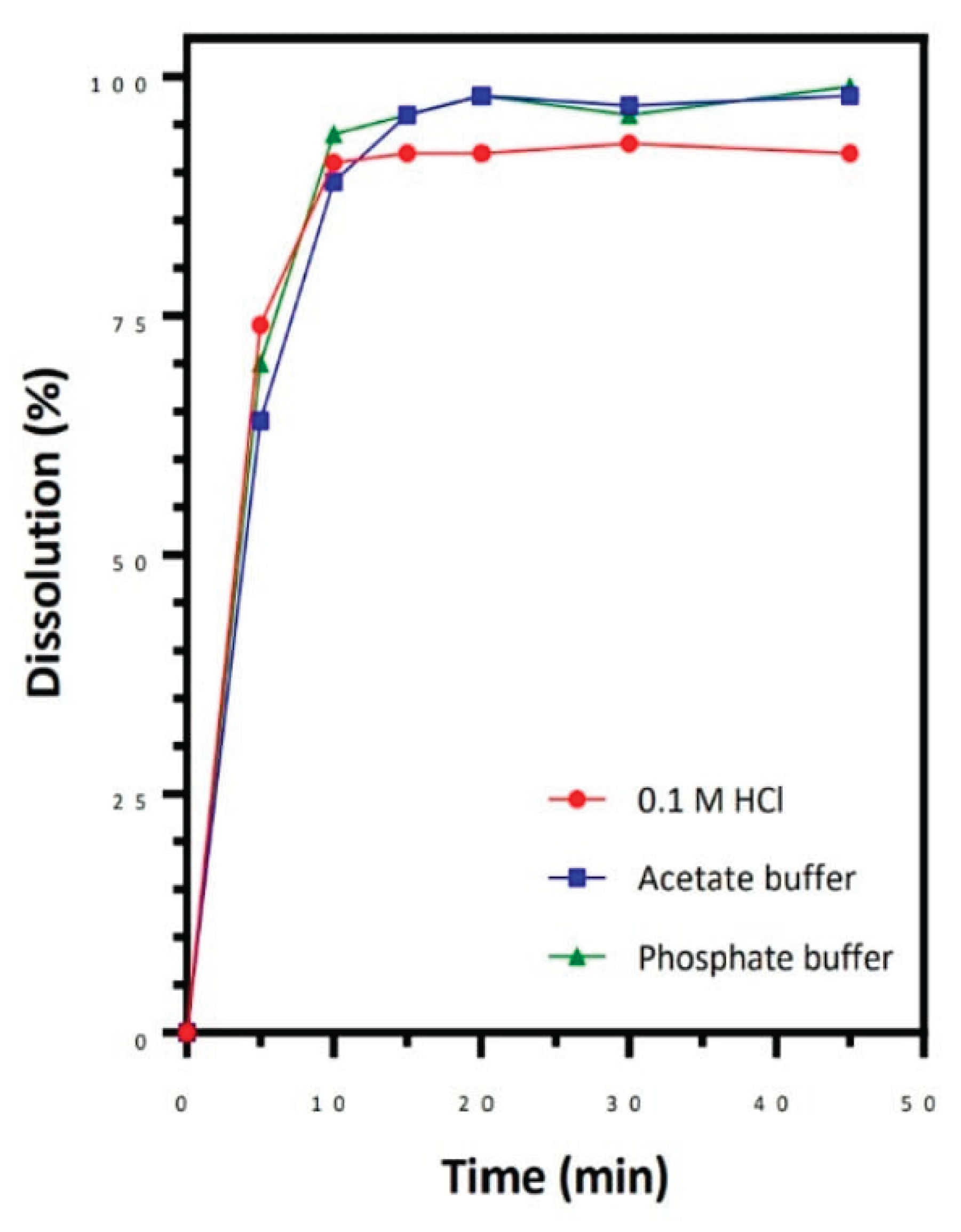
Figure 2.
Dissolution(%) and Time(min) graph.
Figure 2.
Dissolution(%) and Time(min) graph.
A Drug Product's Eligibility For A Biowaiver Based On Bcs
When a single therapeutic dose fails to meet high solubility thresholds but the reference product meets or surpasses such standards under pertinent testing parameters, pharmacokinetic analyses of AUC and Cmax at different doses that include the highest therapeutic quantity offer evidence in favor of BCS-based waivers. Drug products that are absorbed sublingually or buccally do not have a BCS-based biowaiver application. Furthermore, the application of the BCS-based biowaiver technique is limited to situations in which the administration route involves water. If it is also expected that the product will be administered without water, a bioequivalence study should be conducted in which the product is dosed without water (for example, orodispersible products). To be qualified for a BCS-based biowaiver, a medicinal product must fulfill specifications for its excipients and in vitro dissolving ability. The BCS-based biowaiver criteria apply to drug products that depends upon specific conditions including being immediate-release oral systemic dosage forms, having equivalent formulations and strengths as the reference product and meeting solubility and permeability requirements of BCS Class I and III. [
19]
Application of Biopharmaceutics
Therapeutic formulation and design, as well as the assessment of novel therapeutic candidates, are significantly influenced by biopharmaceutics. For example, many medications have problems with stability or solubility, which might hinder proper absorption. To enhance absorption, biopharmaceutical researchers respond by using methods including pH adjustment, particle size reduction, and the creation of prodrugs—inactive substances that the body changes into active medications.
Biopharmaceutics helps the generic medicine industry develop cost-effective substitutes for name-brand drugs without sacrificing efficacy or quality. The strict bioequivalence testing criteria in this industry are crucial to helping patients all across the world receive safe, easily available drugs.
The European Medicines Agency (EMA) and U.S. Food and medication Administration (FDA) use biopharmaceutic data for assessing new medication applications. Biopharmaceutic evaluations confirm medication safety and performance requirements before granting availability to the general public. These rules also mandate testing of medication stability alongside solubility and dissolution rates because both influence how a drug functions in the body
Primary focus of biopharmaceutic research is always patient safety. Researchers can customize therapies to reduce side effects by knowing how different patient populations react to different medications. For example, formulations that take into consideration genetic variations in drug metabolism might be developed using personalized medicine techniques, resulting in safer and more efficient treatments. [
20]
BCS Exclusion
-
1.
Drugs with a Limited Therapeutic Range:
This guideline mentions that products having a narrow therapeutic range designation in their label require specific drug substances that need pharmacodynamic effect monitoring alongside therapeutic drug concentration evaluation. The drug substances digoxin, lithium, phenytoin, theophylline and warfarin represent various BCS type recommendations. All firms must consult with their assigned review division to learn if their product meets the narrow therapeutic range requirements since pharmacodynamic and therapeutic drug concentration monitoring does not automatically classify a drug as part of that framework. [
21]
-
2.
Materials Meant to be absorbable in the Oral Cavity:
The BCS cannot justify waiving necessary in vivo BA/BE tests when delivering drugs through the mouth or via the buccal route or sublingual forms. [
21]
Conculsion
As shown through the provided examples a redefined BCS approach helps assess pharmaceutical substances for oral sustained-release delivery by providing a simple method to identify which drug features are most likely to affect oral absorption. Reorganizing the dissolution number equation allows scientists to calculate the dissolving rate sensitivity across multiple particle sizes. The concept of solubility limited absorbable dose (SLAD) permits analysts that identify doses that can lead to intestinal solubility restricting oral absorption. When designing methods for optimum formulation and identifying prospective critical quality attributes (CQAs), it is also useful to see which aspects are most likely to compromise a drug's in vivo performance. [
22]
References
- Wu F, Cristofoletti R, Zhao L, Rostami-Hodjegan A. Scientific considerations to move towards biowaiver for biopharmaceutical classification system class III drugs: How modeling and simulation can help. Biopharm Drug Dispos. 2021 Apr;42(4):118-127. Epub 2021 Apr 22. PMID: 33759204. [CrossRef]
- Amidon GL, Lennernas H, Shah VP, and Crison JR, “A theoretical basis for a biopharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability Pharm. Res., 1995, 12, 413–420.
- Guidance for industry, “Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system,” CDER/FDA , August 2000.
- Biopharmaceutics Classification System Guidance Office of Pharmaceutical Science, CDER/FDA, August 2006.
- Biopharmaceutical Classification System: Tool based prediction for drug dosage Formulation Ajay Kumar Shukla , Ram Singh Bishnoi, Suresh Kumar Dev, Manish Kumar, Vikas Fenin Mohanlal Sukhadia University Udaipur Rajasthan-313001 India.
- https://www.researchgate.net/publication/324678815.
- SAMINENI R, CHIMAKURTHY J, KONIDALA S. Emerging Role of Biopharmaceutical Classification and Biopharmaceutical Drug Disposition System in Dosage form Development: A Systematic Review. Turk J Pharm Sci. 2022 Dec;19(6):706-713. [CrossRef]
- Waiver of In Vivo Bioavailability and Bioequivalence.
- Studies for Immediate-Release Solid Oral Dosage Forms.
- Based on a Biopharmaceutics Classification System; Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington, DC, August 2000. http://www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/ Guidances/ucm070246.pdf (accessed Jan 11, 2011).
- NEUROQUANTOLOGY | NOVEMBER 2022 | VOLUME 20 | ISSUE 15|PAGE 3165-3177|.
- Jyoti Kumari et al / An updated account of the BCS: Biopharmaceutical Classification System.
- B. Basanta Kumar Reddy and A. Karunakar.
- Regulatory Affair Dpt., Indchemie Health Specialities Pvt. Ltd. (Alkem Research Laboratory), M.I.D.C, Taloja, Dist-Raigarh, Navi Mumbai Maharashtra-410208, India.
- Bioavailability and Bioequivalence- Jake J. Thiessen, Ph.D, University of Toront, Canada, Email jij thiessen@utoronto.ca.
- Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23.
- Rane, S. S.; Anderson, B. D. What determines drug solubility in lipid vehicles: Is it predictable? Adv. Drug Delivery Rev. 2008, 60 (6) 638– 656.
- Bergström, C. A. S.; Wassvik, C. M.; Johansson, K.; Hubatsch, I. Poorly soluble marketed drugs display solvation limited solubility J. Med. Chem. 2007, 50 (23) 5858– 5862.
- Zaki, N. M.; Artursson, P.; Bergström, C. A. S. A modified physiological BCS for prediction of intestinal absorption in drug discovery Mol. Pharmaceutics 2010, 7 (5) 1478– 1487.
- International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use ICH Harmonised Guideline Biopharmaceutics Classification System-Based Biowaivers M9, Final Version, Adopted On 20 November 2019.
- Samineni R, Chimakurthy J, Konidala S. Emerging Role of Biopharmaceutical Classification and Biopharmaceutical Drug Disposition System in Dosage form Development: A Systematic Review. Turk J Pharm Sci. 2022 Dec 21;19(6):706-713. PMID: 36544401; PMCID: PMC9780568. [CrossRef]
- In vitro dissolution methodology, mini-Gastrointestinal Simulator (mGIS), predicts better in vivo dissolution of a weak base drug, dasatinib.
- Kus-Slowinska et al. Solubility, permeability, and dissolution rate of naftidrofuryl oxalate based on BCS criteria. Pharmaceutics. 2020; 12:1238.
- European medicines agency, science medicines health, ICH M9 guideline on biopharmaceutics classification system-based biowaivers, EMA/CHMP/ICH/493213/2018.
- Cook J, Addicks W, Wu YH. Application of the biopharmaceutical classification system in clinical drug development--an industrial view. AAPS J. 2008 Jun;10(2):306-10. Epub 2008 May 24. PMID: 18500563; PMCID: PMC2751386. [CrossRef]
- Blume HH and Schug BS, “The Biopharmaceutics classification system (BCS): Class III drugs better candidates for BA/BE waiver”? Eur. J. Pharm. Sci, 1999,9: 117-121.
- Butler JM, Dressman JB. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010 Dec;99(12):4940-54. PMID: 20821390. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).