Submitted:
06 June 2025
Posted:
09 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Normal Autonomic Physiology
- Baroreflex arc: regulates blood pressure via mechanoreceptor-mediated control of heart rate and vascular tone.
- Vagal nerve: modulates parasympathetic output to the heart and GI tract.
- Sympathetic chain: mediates norepinephrine-driven vasoconstriction and cardiac output.
Proposed Mechanisms in Post-COVID Dysautonomia
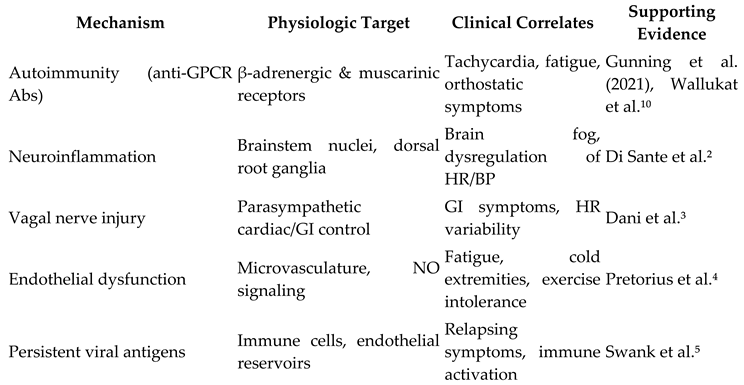
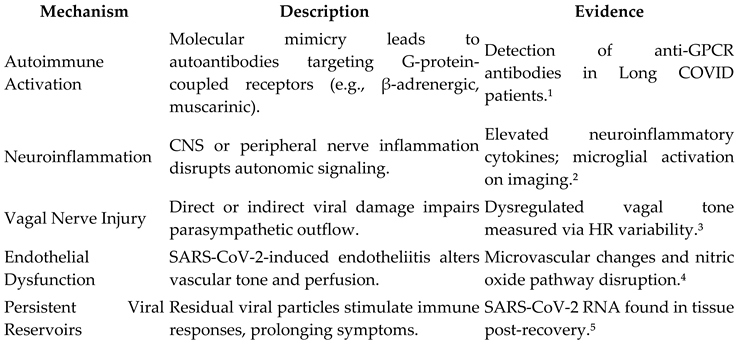
Clinical Manifestations
- Orthostatic Intolerance: lightheadedness, palpitations, dizziness upon standing
- Tachycardia: sustained HR increase ≥30 bpm within 10 minutes of standing (POTS criteria)
- Fatigue and Malaise: often disabling and worsened by exertion (post-exertional malaise)
- Cognitive Impairment (“Brain Fog”): difficulty concentrating, memory lapses
- Gastrointestinal Symptoms: nausea, bloating, delayed gastric emptying
- Temperature Dysregulation: hot flashes, cold extremities
- Sleep Disturbance: insomnia, non-restorative sleep
Diagnostic Approaches
- 10-minute standing test: HR and BP measured supine and standing; HR increase ≥30 bpm with minimal BP drop supports POTS.
- Tilt-table testing: standard for diagnosing orthostatic intolerance and neurocardiogenic syncope.
- Heart rate variability analysis: evaluates vagal tone and autonomic balance.
- QSART (Quantitative Sudomotor Axon Reflex Test): assesses sympathetic sweat gland function.
- Serologic markers: under investigation (e.g., anti-GPCR antibodies).
Therapeutic Strategies
Nonpharmacologic Approaches
- Hydration and Salt Loading: 2–4L fluid/day and up to 10g salt intake improves blood volume.
- Compression Garments: reduce venous pooling.
- Exercise Rehabilitation: starting with horizontal exercise (recumbent bike, rowing) and gradual progression.
Pharmacologic Options
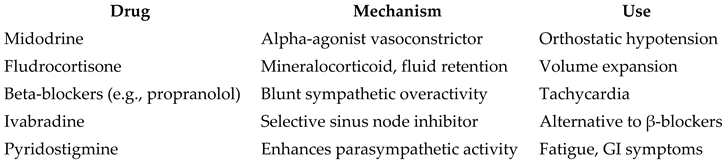
Controversies and Knowledge Gaps
- Autoimmunity vs central sensitization: Are symptoms immune-mediated or centrally driven?
- Lack of biomarkers: Limits diagnostic specificity and treatment targeting.
- Overlap with ME/CFS: Shared symptomology raises questions about shared pathophysiology.
- Sex Disparities: Why are young women disproportionately affected?
- Heterogeneity: No single phenotype or treatment protocol fits all patients.
Future Research Directions
- Longitudinal cohort studies to map symptom trajectory and resolution.
- Biomarker discovery (e.g., cytokines, antibodies, HR variability).
- Immunophenotyping to clarify autoimmune contributions.
- Clinical trials of pharmacologic and rehabilitation interventions.
- Neuroimaging to visualize brainstem and autonomic centers.
Conclusion
Author Contributions
Funding
Conflict of Interest
Use of AI Tools
References
- Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome following COVID-19. Heart Rhythm. 2021;18(9):1760-1765. [CrossRef]
- Di Sante G, Buonsenso D, Rose CD, et al. Immune profile of children with post-acute sequelae of SARS-CoV-2 infection (Long COVID). Cell Death Dis. 2021;12(10):1-9. [CrossRef]
- Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63-e67. [CrossRef]
- Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. [CrossRef]
- Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis. 2023;76(1):e487-e490. [CrossRef]
- Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. [CrossRef]
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205-211. [CrossRef]
- Raj SR, Guzman JC, Harvey P, et al. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can J Cardiol. 2020;36(3):357-372. [CrossRef]
- Miglis MG, Prieto T, Shaik R, Muppidi S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30(5):449-451. [CrossRef]
- Wallukat G, Schimke I. Agonistic autoantibodies directed against G-protein–coupled receptors and their relationship to cardiovascular diseases. Front Biosci (Elite Ed). 2014;6:264-278. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



