1. Introduction
Radial nerve palsy is a peripheral neuropathy characterized by motor deficits, primarily manifesting as wrist drop and weakness of finger extension, often accompanied by sensory disturbances over the dorsum of the hand. The most common pathophysiology involves neurapraxia secondary to external compression of the radial nerve, typically occurring during prolonged immobilization or mechanical pressure[
1,
2].
Compression leads to mechanical deformation, impaired microvascular perfusion, and subsequent demyelination, which contribute to the clinical manifestations of nerve dysfunction[
2]. Although many cases recover spontaneously with conservative management, the extended duration of functional impairment—such as wrist drop—can result in temporary disability, decreased quality of life, and significant socioeconomic burden, particularly in modern working populations.
An important anatomical factor contributing to the vulnerability of the radial nerve is its course through the spiral groove of the humerus. The spiral groove is a well-known anatomical depression on the posterior aspect of the humerus through which the radial nerve and deep brachial artery pass[
3]. Due to its superficial location and confinement between muscular and bony structures, the radial nerve at this site is particularly prone to compression injuries, explaining the frequent occurrence of conditions such as Saturday night palsy. Typical mechanisms of compression at the spiral groove include direct pressure from leaning the arm against a hard surface for extended periods (e.g., during sleep or prolonged immobilization), compression from external forces or tight clothing, or, less commonly, compression due to humeral fractures or masses[
4].
In response to the limited effectiveness and prolonged recovery associated with conservative treatment of radial nerve palsy, ultrasound (US)-guided hydrodissection (HD) has emerged as a promising minimally invasive therapeutic option. According to Gragossian et al. (2025), patients typically recover 4 months after starting treatment, provided the nerve is not lacerated or torn, highlighing the slow course of spontaneous recovery[
3]. Ultrasonography has been identified as the preferred modality for interventions around the spiral groove, and HD of the radial nerve near this region is a technique used to separate the nerve from surrounding tissues under ultrasound guidance by injecting a fluid (usually saline, sometimes with anesthetics or corticosteroids) [
5]. This technique enables real-time visualization and mechanical decompression of the nerve. Gill et al. (2022) reported that US-guided HD provides complete symptom resolution in radial tunnel syndrome, suggesting that this technique may offer a safe and effective alternative to surgical intervention in select cases of compressive neuropathy[
6]. Dextrose 5% in water (D5W), when used as the primay injectate, appearss to contribute additonal therapeutic benefit to that of a mechanical hydrodissection effect alone, outperforming hydrodissection with saline or triemcinollone in the treatment of carpal tunnel syndrome [
7,
8].
When a patient presents with acute weakness involving a peripheral nerve, the diagnostic question is whether the nerve dysfunction or injury represents neurapraxia, neurotmesis, or axonotmesis. It is commonly taught that in such cases, a conduction block prevents the distinction between these three significantly different levels of damage through immediate examination or prompt electromyographic evaluation. We present a case of radial nerve palsy presumed to result from compression at the spiral groove, successfully treated with US-guided HD using D5W without local anesthetics (LA). The outcome of this case suggests that acute weakness following apparent peripheral nerve injury may provide an opportunity to confirm neurapraxia as the nature of the injury and determine the degree of its prompt reversibility. This, in turn, may minimize or avoid loss of work time, alter formal rehabilitation programming, and favorably influence long-term prognosis.
2. Case Presentation
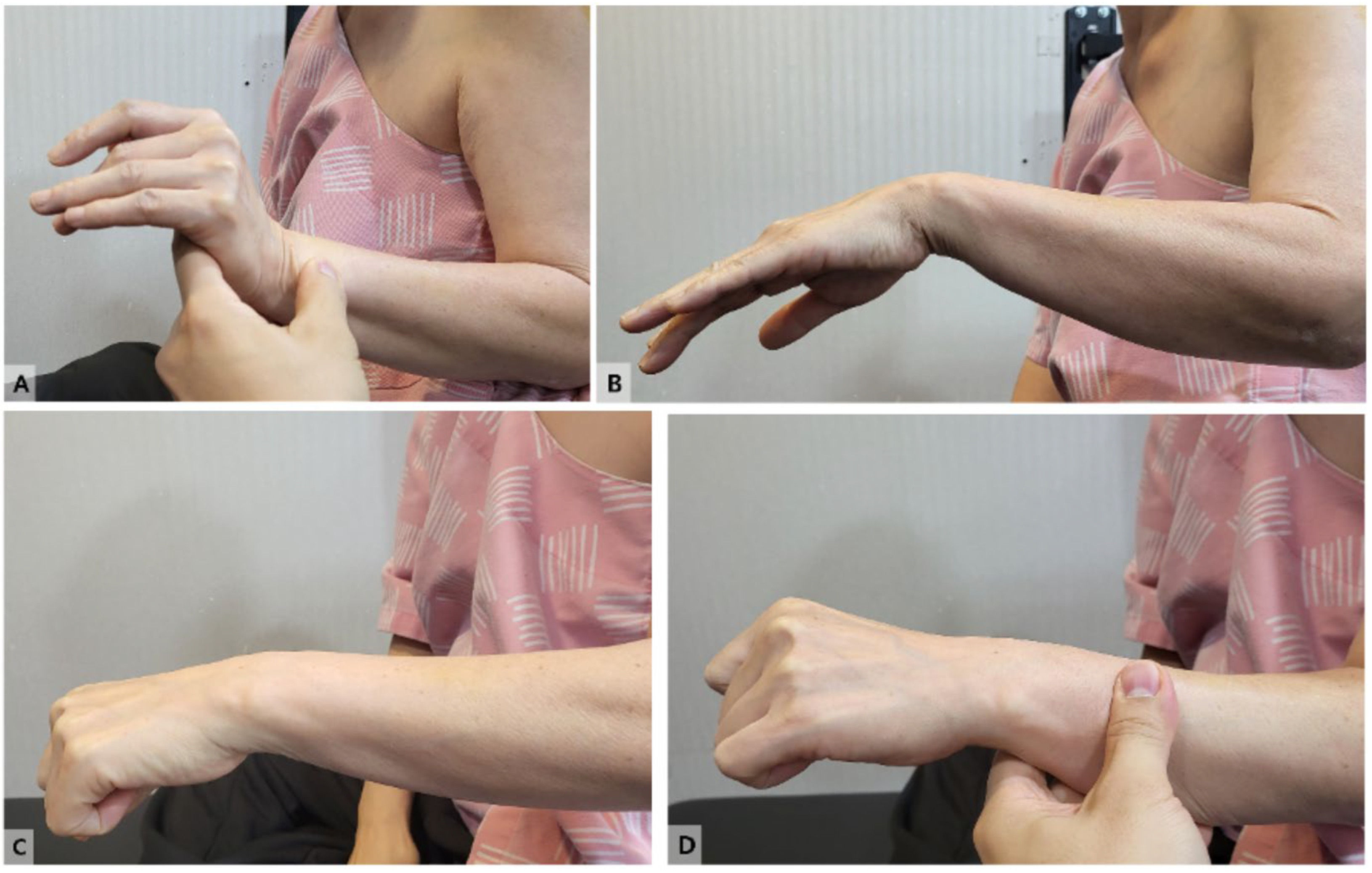
A 54-year-old Korean woman presented with a sudden onset of left wrist drop that developed earlier that morning. On physical examination, the patient was unable to actively extend her left wrist (
Figure 1 and Video 1).
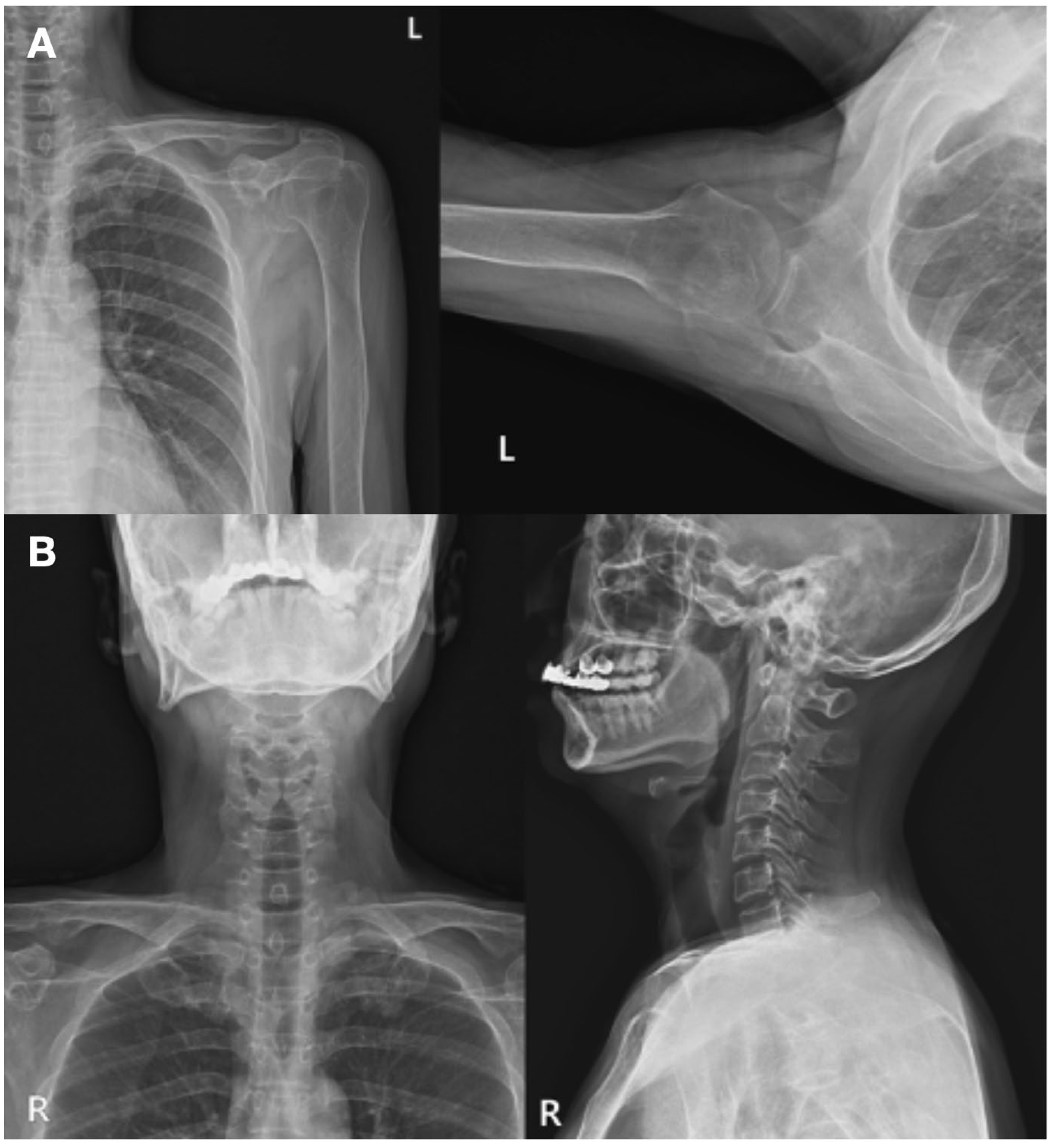
The patient had a history of poorly controlled diabetes mellitus, managed with insulin therapy. No other underlying medical conditions were reported. The patient denied any previous history of similar symptoms or recent trauma. Plain radiographs of the upper extremity revealed no bony deformities or other structural abnormalities that could account for the neurological deficits (
Figure 2A, 2B).
Neurological examination was conducted using the Medical Research Council (MRC) scale, revealing preserved strength, with grade IV or higher in the shoulder abductors, elbow flexors, and extensors. Sensory examination did not identify any paresthesia or hypoesthesia in the affected limb.
Given the clear clinical findings suggestive of radial nerve palsy, particularly in the context of poorly controlled diabetes as a potential predisposing factor, US-guided HD with D5W without LA was recommended. This decision followed a thorough discussion of other conservative treatment options, and the patient provided consent for the procedure.
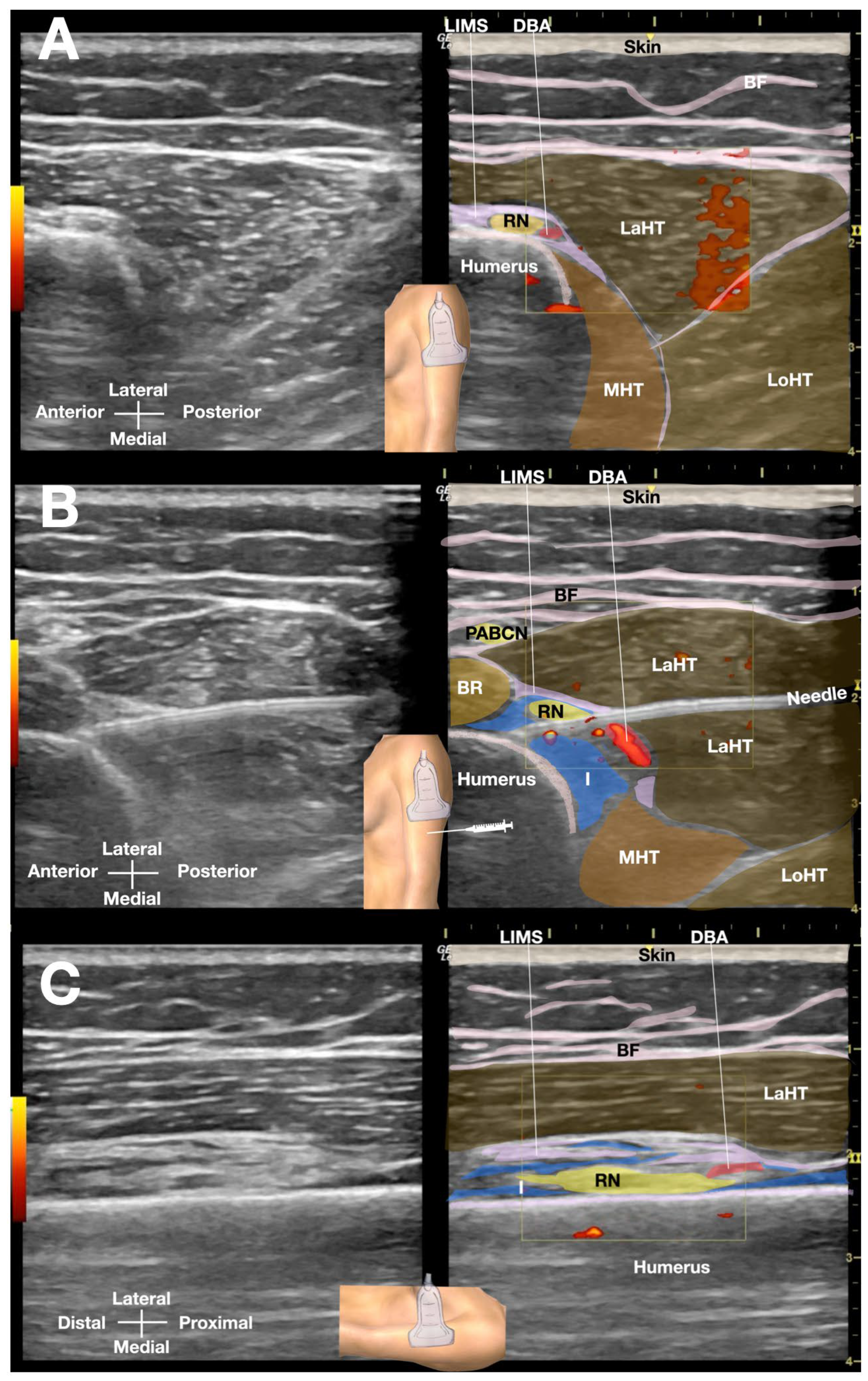
The patient was positioned side-lying for the procedure, targeting the proximal spiral groove using a posterior-to-anterior in-plane needle approach. After numbing the skin with 1% lidocaine, a total of 50 mL of D5W with no LA was injected under real-time sonographic guidance to hydrodissect the radial nerve the spiral groove (
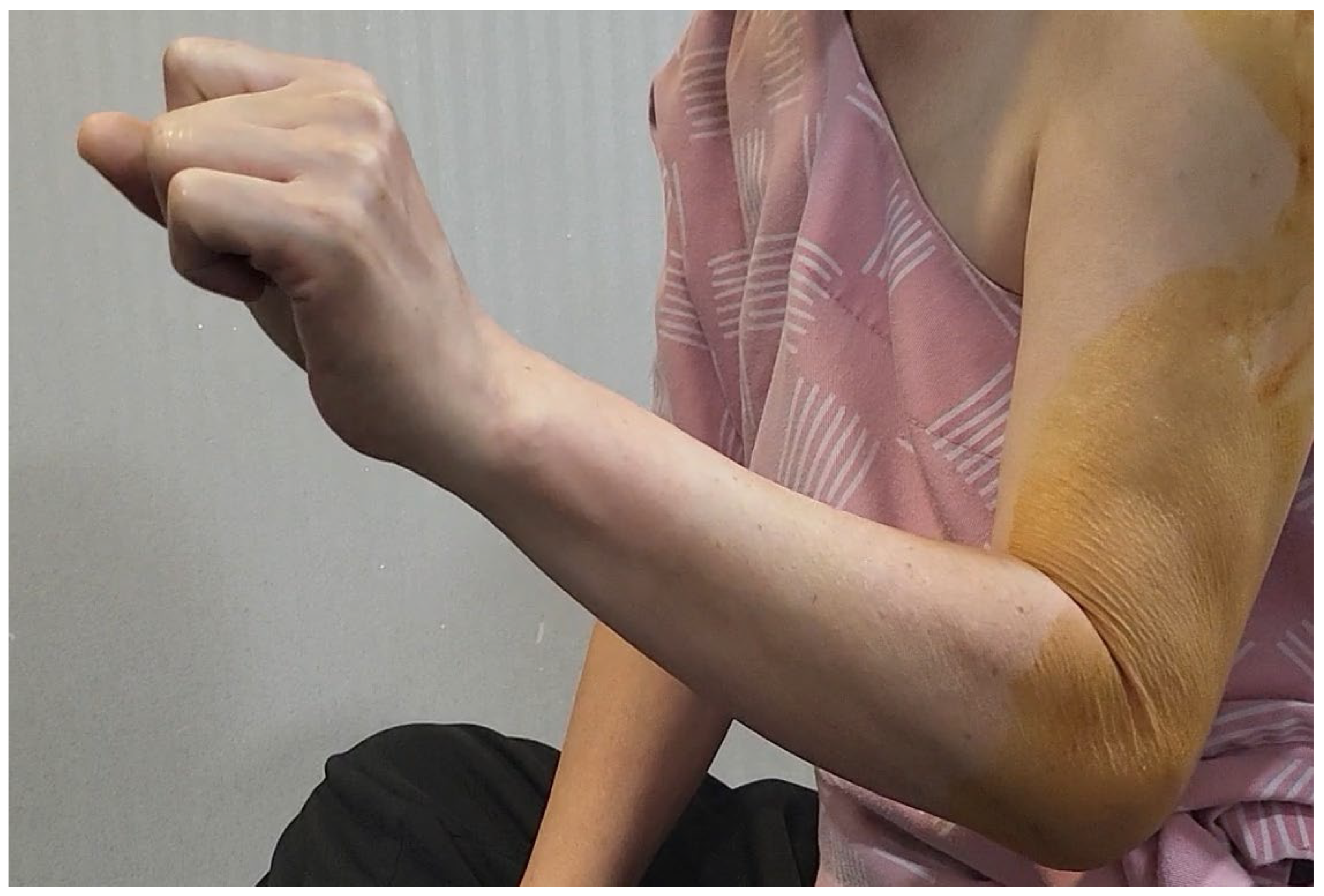
Figure 3 or Video 2). Immediate post-procedural assessment revealed marked improvement in wrist extension, indicating a positive therapeutic response (
Figure 4 and Video 3).
In a follow-up telephone interview conducted one month after the procedure, the patient reported significant improvement in wrist drop and extensor pollicis longus (EPL) dysfunction following US-guided HD. At that time, she indicated no residual impairment in wrist function and described complete restoration of normal daily activities. During a three-month follow-up telephone interview, the patient reported continued full restoration of left wrist function and strength. She noted no disturbances to her daily activities due to the episode of wrist drop from radial nerve palsy.
This retrospective case report was exempt from Institutional Review Board (IRB) review, as it involved the analysis of existing clinical data and documents without the collection or use of personally identifiable information. Written informed consent was obtained for treatment and for publication of clinical details and outcomes through telephone interviews. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
3. Minireview of the Management of the Acute Radial Nerve Palsy
3.1. Definition and Pathophysiology
Radial nerve palsy represents a distinct peripheral neuropathy resulting from compression, traction, or direct trauma to the radial nerve, with the spiral groove of the humerus being the most frequent site of injury [
9]. The nerve’s anatomical course through this narrow osseous groove, where it is tethered between the lateral intermuscular septum and the humeral periosteum, creates a predisposition to compression injuries [
10,
11]. Pathophysiologically, the condition may manifest as neurapraxia (reversible conduction block), axonotmesis (axonal disruption with intact connective tissue), or neurotmesis (complete nerve transection), with significant implications for prognosis and management [
12,
13,
14].
3.2. Epidemiology and Risk Factors
Population-based studies indicate radial nerve injuries account for approximately 15-20% of all peripheral nerve trauma [
15]
. Most patients with radial nerve palsy are from the working population, and about 70.1% are male. The primary cause of these injuries is trauma, with humeral shaft fractures constituting the most common etiology (29.9%), particularly middle-third spiral fractures (Holstein-Lewis variant), followed by iatrogenic causes (17.7%) including surgical positioning and orthopedic procedures [
16]. Other causes include postoperative fibrosis [
5] (17.7%), lacerations (17.7%), and compressive neuropathies (“Saturday night palsy,” “crutch palsy”) account for 15-20% of cases [
4]. Systemic conditions such as diabetes mellitus and alcaholism represent important non-traumatic risk factors for radial nerve palsy [
9]. This underscores the need for healthcare professionals to be aware of non-traumatic causes of radial nerve injuries, especially when there are no obvious external injuries.
3.3. Clinical Presentation and Diagnostic Evaluation
The classic triad of wrist drop, finger extension weakness, and preserved elbow extension (triceps function) localizes the lesion to the spiral groove [
9,
17]. Sensory disturbances, when present, typically involve the dorsal radial aspect of the hand but spare the posterior arm due to preservation of the posterior cutaneous nerve of the arm [
18]. Electrodiagnostic studies remain the gold standard for evaluation, with motor conduction studies showing reduced amplitude across the spiral groove and needle EMG demonstrating fibrillations in radial-innervated muscles distal to the injury site [
19]. High-resolution ultrasonography (HRUS) (12-18 MHz transducer) provides dynamic assessment of nerve swelling, fascicular pattern disruption, and perineural fibrosis, with a sensitivity of 89% for compressive lesions [
20].
Dynamic Assessment: HRUS allows for real-time observation of nerve structures, enabling the identification of changes in nerve size and shape during movements or palpation [
21].
Nerve Swelling: HRUS can detect nerve swelling by measuring the cross-sectional area of the nerve, which can be a sign of inflammation or injury [
20].
Fascicular Pattern Disruption: HRUS can visualize the fascicular honeycomb pattern of nerves, and any disruptions in this pattern, or excessive swelling of any particular fascicle(s) can indicate edema in the fascicle(s), signifying nerve damage or compression [
20].
Perineural Fibrosis: HRUS can detect the presence and extent of fibrosis, which is the thickening of the perineurium (the outer sheath of the nerve) and can be a sign of chronic nerve damage [
5,
20]. Shear wave elastography, a specific feature of the ultrasound, can help delineate the increase in hardness of the scarring or fibrosis within the nerve or in the surrounding soft tissues of the involved nerve [
5].
Sensitivity for Compressive Lesions: The sensitivity of 89% for compressive lesions means that HRUS can accurately detect a high percentage of nerve compression cases, making it a valuable tool for diagnosis and management [
20].
Complementary Tool: HRUS serves as a complementary tool to other imaging modalities and electrodiagnostic studies, providing detailed anatomical information about the nerves[
20,
21].
Clinical Applications: HRUS can be used for diagnosing nerve entrapments like carpal tunnel syndrome[
20,
22], as well as evaluating traumatic nerve injuries, and tumors.
3.4. Therapeutic Interventions
Conservative management, indicated for closed injuries without evidence of transection, incorporates wrist extension splinting (15-30° of extension) to prevent contractures while allowing metacarpophalangeal joint mobility [
9,
23,
24,
25]. Nerve gliding exercises, initiated at 3-4 weeks post-injury, have demonstrated improved functional outcomes in randomized trials [
23,
24]. For other common peripheral nerve entrapment syndrome, such as carpal tunnel syndrome, ultrasound-guided hydrodissection with 5% dextrose has shown particular promise, with a 2023 systematic review and meta-analysis documenting that ultrasound-guided hydrodissection of the median nerve with D5W outperformed control injection including steroid, and hydrodissection with high-volume D5W was superior to that with low-volume D5W [
7]. Hydrodissection with D5W may result in a clinically important and durable benefit in those experiencing persistent or recurrent CTS after surgery [
26]. There has not been a large randomized controlled trial studying the efficacy of D5W hydrodissection in patients with radial nerve palsy. Only one case report has indicated that ultrasound-guided perineural injection with D5W may be an effective [
27] and novel intervention for radial nerve palsy, while another similar study used platelet-rich plasma for radial nerve palsy[
28]. However, these case reports involved patients who had failed conservative treatment.
3.5. Surgical Considerations and Prognosis
For refractory cases that do not respond to conservative treatments, indicating no discernible nerve regeneration, surgical options may be considered to restore wrist and finger functions. These include tendon transfers such as, pronator teres to extensor carpi radialis longus, flexor carpi radialis to extensor digitorum communis, palmaris longus to extensor pollicis longus [
29]. Surgical exploration is also warranted for open injuries, progressive deficits, or failure of conservative management beyond 3-6 months [
9,
30]. Intraoperative nerve action potential recordings guide decision-making, with neurolysis performed for preserved conduction versus grafting for absent potentials[
31]. Prognostically, neurapraxic injuries typically resolve within 2-3 month[
14], while axonotmetic lesions require 3-6 months for regeneration (1-2 mm/day) [
32,
33]. Poor prognostic indicators include complete motor/sensory loss at presentation (Sunderland grade IV/V: this classification indicates a severe nerve injury where the nerve is either completely severed (grade V) or has significant damage with a risk of complete loss of function (grade IV). This means there is a lack of nerve regeneration and a less optimistic prognosis for recovery.) [
9,
34] and advancing patient age (>50 years: Older patients may have a harder time recovering from nerve injuries due to factors like reduced nerve regeneration capacity and a higher risk of co-existing medical conditions that can interfere with healing.)[
30]. Severe Initial Weakness: The degree of initial weakness is a significant factor. If the initial weakness is severe, it suggests a more significant nerve injury, which may be harder to recover from [
9,
30,
34].
Denervation Findings on EMG: Electromyography (EMG) is a test that measures the electrical activity of muscles. Denervation refers to a loss of nerve supply to a muscle. Denervation findings on EMG can indicate severe nerve damage and a poor prognosis [
34]. Other Factors: While age and initial presentation are key, other factors like the underlying cause of the palsy, the specific location of the injury, and the patient’s overall health can also influence the prognosis [
9,
30,
34]. In summary, a complete loss of function at the time of diagnosis, an older age, and severe initial weakness or denervation findings on EMG are all indicators of a poorer prognosis for radial nerve palsy.
3.6. Emerging Concepts and Future Directions
Recent advances include the application of diffusion tensor imaging for nerve integrity assessment [
35] and the development of nerve-specific rehabilitation protocols [
36]. Ongoing clinical trials are evaluating the role of platelet-rich plasma (PRP) injections [
28] and low-intensity pulsed ultrasound in accelerating nerve recovery[
37]. These developments underscore the evolving paradigm in radial nerve palsy management, emphasizing early precise diagnosis and targeted intervention to optimize functional outcomes. However, ultrasound-guided hydrodissection of the radial nerve in the spiral grove with D5W and PRP has not been studied in randomized control trials. There is currently no study on the use of US-guided HD with D5W without LA as a diagnostic and therapeutic intervention for acute radial nerve palsy.
4. Discussion
This case is unique in demonstrating an acute compressive radial nerve palsy that was rapidly and completely reversed by a single US-guided HD injection in an outpatient setting. Such nontraumatic “Saturday night palsy” compressive neuropathies typically recover only gradually over several weeks to months with conservative management [
38], making the immediate restoration of function in our patient especially remarkable. The use of high-resolution ultrasound allowed prompt identification of the radial nerve at the spiral groove and targeted perineural injection, exemplifying the expanding role of ultrasound in both diagnosing and treating peripheral nerve entrapments [
39]. Notably, the injectate was D5W without any LA, allowing for the immediate demonstration of the full extent of motor responsiveness to nerve compression relief after injection. This enables adjustments in work ability and modifications to rehabilitation approaches, assisting the rehabilitation team with planning. In contrast to prior reports where HD was applied only after weeks of unsuccessful conservative therapy [
27], our case suggests that immediate US-guided HD at presentation can dramatically improve function even in acute neuropathy.
Standard treatment for radial nerve palsy ranges from conservative management to surgical intervention, each with limitations. For compressive or closed-injury neuropathies, initial management is typically conservative (observation, splinting, and physical therapy) since up to 95% of closed-injury radial palsies will eventually recover fully without surgery, albeit over a highly variable timeframe of about 3 to 68 months [
40]. During this prolonged recovery period, patients endure significant functional deficits (wrist and finger drop with impaired hand function) and there is no guarantee of complete recovery. Surgical exploration and decompression (or nerve repair) is usually reserved for cases with a high suspicion of nerve laceration/entrapment or for palsies that show no improvement after an appropriate observation period; immediate surgery is typically undertaken only in the setting of open injuries or clear transection evidence [
40]. Thus, the conventional approach often involves a period of watchful waiting, which can prolong disability and may still result in the need for complex surgical or tendon transfer procedures if recovery plateaus.
US -guided HD has emerged in recent years as a promising minimally invasive treatment for peripheral nerve entrapments, aimed at mechanically separating the nerve from surrounding tissues to relieve compression. Growing evidence supports its efficacy in a variety of neuropathies. For example, Gill et al. (2022) reported that all patients with radial tunnel syndrome in their series achieved complete and lasting symptom resolution after US-guided HD, with none requiring surgery.[
6] In their literature review, 61 additional patients with radial nerve pathology treated by HD were identified, all of whom showed significant clinical improvement, with five studies reporting over 90% symptom relief and no major adverse effects. Likewise, Chen et al. (2018) described a 62-year-old woman with compressive radial nerve palsy who experienced substantial motor and sensory recovery after two sessions of D5W HD, following two months of failed conservative management[
27].
Beyond the radial nerve, research indicates that HD can benefit other entrapment neuropathies. In carpal tunnel syndrome (median nerve compression), randomized trials have demonstrated that perineural injection of 5% dextrose yields greater symptom improvement and reduction in median nerve swelling than control treatments [
7,
8,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52]. Similarly, case reports have documented successful US-guided HD in ulnar nerve entrapment at the elbow and in other nerve compression syndromes [
6]. Collectively, these findings suggest that perineural HD can effectively alleviate nerve compression and improve function in select patients, potentially serving as a surgery-sparing alternative in the management of compressive neuropathies [
6,
27,
43].
Despite the favorable outcome, this report has several limitations. The most significant limitation is the absence of electrodiagnostic studies (nerve conduction studies and electromyography). While ultrasound supported the diagnosis of a focal radial neuropathy, electrodiagnostic testing is crucial for confirming the diagnosis, localizing the site of compression, and assessing the severity and type of nerve injury. Without these studies, we cannot definitively rule out other potential causes of wrist drop or objectively quantify the degree of nerve recovery. Although ultrasound supported the diagnosis of a focal radial neuropathy and electromyography has limited sensitivity in the first 2–3 weeks of an acute nerve injury [
38], the absence of nerve conduction data means that our assessment of improvement relied solely on clinical examination.
Second, as this is an isolated case without a control group, we cannot definitively exclude the possibility of spontaneous recovery or placebo effect. The timing of the recovery – occurring immediately after the HD – strongly suggests a true therapeutic benefit, and importantly no neural blockade medication was used (avoiding the confounder of a temporary anesthetic effect). Nonetheless, one case cannot prove causation, and the natural history of acute compressive radial palsy is variable.
Finally, the generalizability of this observation is limited. A single successful outcome does not guarantee that US-guided HD will work for all cases of radial nerve palsy; factors such as the severity of nerve injury (neurapraxia vs. axonotmesis), chronicity, or anatomical variations could influence efficacy. While generally considered safe, US-guided HD carries potential risks, including infection, bleeding, nerve injury, and allergic reaction to the injectate. These risks can be minimized through careful hydrodissection techniques, ultrasound real-time guidance, and the absence of neural blocking medication, as demonstrated in this case.
In addition to the interventions discussed, alternative and complementary therapies for radial nerve palsy include occupational therapy to improve hand function and activities of daily living, nerve gliding exercises to promote nerve mobility, and orthotic splinting to prevent contractures. Further research - ideally in the form of larger case series or controlled trials - is needed to validate the efficacy of US-guided HD, optimize the technique (e.g., timing, injectate volume, with or without LA), and identify which patients with compressive radial neuropathy are most likely to benefit from this approach.
4.1. Lessons Learned from this Case Report
Early Intervention: This case suggests that early intervention with US-guided HD in acute compressive radial nerve palsy may lead to rapid and complete recovery, potentially avoiding the prolonged disability associated with conservative management. Additionally, it diagnostically supports the primary pathology of neurapraxia, which is largely reversible.
Importance of Ultrasound: High-resolution ultrasound is valuable for both diagnosing and guiding treatment of peripheral nerve entrapments like radial nerve palsy.
D5W as a Safe Injectate: The use of 5% dextrose in water without local anesthetic appears to be a safe and effective injectate for hydrodissection, minimizing the risk of nerve block and allowing for true neural decompression.
Need for Further Research: While this case is promising, it highlights the need for further research, including controlled trials and electrodiagnostic confirmation, to validate the efficacy of US-guided HD and identify appropriate candidates for this intervention.
5. Conclusions
In summary, we present an acute radial nerve palsy that was rapidly relieved with US-guided HD, highlighting an innovative approach to decompressing a peripheral nerve in real time. The key clinical insight is that early US evaluation and guided HD using an innocuous injectate (5% dextrose water) without LA can lead to immediate and sustained neurologic improvement in a compressive neuropathy.
To strengthen the evidence base for this treatment, further rigorous research is essential. We recommend conducting large case series or controlled studies to accurately assess the true efficacy of US-guided HD for radial nerve palsy. Future studies should focus on optimizing injection techniques including timing, volume, and the choice of injectate, while also identifying specific patient selection criteria.
Key factors for selecting patients who might benefit most from US-guided HD include the duration of symptoms, the severity of nerve injury (neurapraxia vs. axonotmesis), and the presence of underlying conditions such as diabetes. Until more comprehensive data are available, US-guided HD should be considered an investigational option for the management of compressive radial neuropathy.
Author Contributions
Conceptualization, H.W.L., Y.H.Y., D.C.J.S., T.S., and K.H.S.L.; methodology, H.W.L., Y.H.Y., K.D.R. and K.H.S.L.; software, J.H.W., C.P., M.J.L., Y.H.Y., and K.H.S.L.; validation, H.W.L., J.H.W., C.P., M.J.L., Y.H.Y., Y.S.S., H.Y., R.P., J.S., J.A., D.C.J.S., T.S., K.D.R. and K.H.S.; writing—original draft preparation, H.W.L., J.H.W., C.P., Y.H.Y., and K.H.S.L.; writing—review and editing, H.W.L., J.H.W., C.P., M.J.L., Y.H.Y., Y.S.S., H.Y., R.P., J.S., J.A., D.C.J.S., T.S., K.D.R. and K.H.S.L.; visualization, H.W.L., Y.H.Y., D.C.J.S., T.S., K.D.R. and K.H.S.L.; supervision, Y.H.Y., D.C.J.S., K.D.R. and K.H.S.L.; project administration, H.W.L., J.H.W., C.P., M.J.L., Y.H.Y., Y.S.S., H.Y., R.P., J.S., J.A., D.C.J.S., T.S., K.D.R. and K.H.S.L.; funding acquisition, H.W.LY.H.Y., Y.S.S., D.C.J.S., T.S., K.D.R. and K.H.S.L. All authors have read and agreed to the published version of the manuscript.”
Funding
Please add: “This research received no external funding”
Institutional Review Board Statement
This retrospective case report was exempt from Institutional Review Board (IRB) review, as it involved the analysis of existing clinical data and documents without the collection or use of personally identifiable information. Written informed consent was obtained for treatment and for publication of clinical details and outcomes through telephone interviews. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study. Written informed consent has been obtained from the patient to publish this paper
Data Availability Statement
Data is included in the manuscript.
Acknowledgments
Due to the same amount of contribution, H.W.L., Y.H.Y., and K.H.S.L. should be the co-first-author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shao, Y.C., et al., Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br, 2005. 87(12): p. 1647–52.
- Mackinnon, S.E., Pathophysiology of nerve compression. Hand Clin, 2002. 18(2): p. 231–41.
- Saran, S., et al., Unveiling the spiral groove: a journey through clinical anatomy, pathology, and imaging. Clin Radiol, 2024. 79(11): p. 799–804. [CrossRef]
- Rasulić, L., et al., Etiological and epidemiological characteristics of surgically treated radial nerve lesions: A 20-year single-center experience. Front Surg, 2022. 9: p. 942755. [CrossRef]
- Su, D.C., K.V. Chang, and S.K.H. Lam, Shear Wave Elastography to Guide Perineural Hydrodissection: Two Case Reports. Diagnostics (Basel), 2020. 10(6). [CrossRef]
- Gill, B., R. Rahman, and M. Khadavi, Ultrasound-Guided Hydrodissection Provides Complete Symptom Resolution in Radial Tunnel Syndrome: A Case Series and Scoping Review on Hydrodissection for Radial Nerve Pathology. Curr Sports Med Rep, 2022. 21(9): p. 328–335. [CrossRef]
- Lam, K.H.S., et al., Ultrasound-Guided Interventions for Carpal Tunnel Syndrome: A Systematic Review and Meta-Analyses. Diagnostics (Basel), 2023. 13(6). [CrossRef]
- Buntragulpoontawee, M., et al., The Effectiveness and Safety of Commonly Used Injectates for Ultrasound-Guided Hydrodissection Treatment of Peripheral Nerve Entrapment Syndromes: A Systematic Review. Front Pharmacol, 2020. 11: p. 621150. [CrossRef]
- Gragossian, A. and M.A. Varacallo, Radial Nerve Injury, in StatPearls. 2025, StatPearls Publishing.
- Copyright © 2025, StatPearls Publishing LLC.: Treasure Island (FL).
- Fleming, P., et al., One-third, two-thirds: relationship of the radial nerve to the lateral intermuscular septum in the arm. Clin Anat, 2004. 17(1): p. 26–9.
- Latef, T.J., et al., Injury of the Radial Nerve in the Arm: A Review. Cureus, 2018. 10(2): p. e2199. [CrossRef]
- Kamble, N., D. Shukla, and D. Bhat, Peripheral Nerve Injuries: Electrophysiology for the Neurosurgeon. Neurol India, 2019. 67(6): p. 1419–1422. [CrossRef]
- Matos Cruz AJ, D.J.O., Neurotmesis. StatPearls, 2025.
- Carballo Cuello CM, D.J.O. Neurapraxia. . 2023; Available from: https://www.ncbi.nlm.nih.gov/books/NBK560501/.
- Harhaus, L., et al., Clinical Practice Guideline: The Treatment of Peripheral Nerve Injuries. Dtsch Arztebl Int, 2024. 121(16): p. 534–538.
- Ekholm, R., et al., Primary radial nerve palsy in patients with acute humeral shaft fractures. J Orthop Trauma, 2008. 22(6): p. 408–14. [CrossRef]
- Hsu, P.C., et al., Acute Radial Neuropathy at the Spiral Groove Following Massage: A Case Presentation. Pm r, 2017. 9(10): p. 1042–1046. [CrossRef]
- Glover NM, B.A., Murphy PB. Anatomy, Shoulder and Upper Limb, Radial Nerve. . 2023; Available from: https://www.ncbi.nlm.nih.gov/books/NBK534840/.
- Ramani PK, L.F., Arya K. . Nerve Conduction Studies and Electromyography. . 2025; Available from: https://www.ncbi.nlm.nih.gov/books/NBK611987/.
- Koenig, R.W., et al., High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus, 2009. 26(2): p. E13. [CrossRef]
- Zaottini, F., et al., High-resolution ultrasound of peripheral neuropathies in rheumatological patients: An overview of clinical applications and imaging findings. Front Med (Lausanne), 2022. 9: p. 984379. [CrossRef]
- Elshewi, I.E., et al., Value of ultrasound assessment for traumatic nerve injury of the upper limb. Journal of Ultrasound, 2023. 26(2): p. 409–421. [CrossRef]
- Moscony, A.M.B., 24 - Peripheral Nerve Problems, in Fundamentals of Hand Therapy (Second Edition), C. Cooper, Editor. 2014, Mosby: St. Louis. p. 272–311.
- Pitts, G. and S.C. Umansky, Chapter 8 - Radial Nerve Compression, in Hand and Upper Extremity Rehabilitation (Third Edition), S.L. Burke, et al., Editors. 2006, Churchill Livingstone: Saint Louis. p. 109–120.
- Granhaug, K.B., Chapter 18 - Splinting the Upper Extremity of a Child, in Hand Function in the Child (Second Edition), A. Henderson and C. Pehoski, Editors. 2006, Mosby: Saint Louis. p. 401–431.
- Chao, T.C., et al., The Effectiveness of Hydrodissection with 5% Dextrose for Persistent and Recurrent Carpal Tunnel Syndrome: A Retrospective Study. J Clin Med, 2022. 11(13). [CrossRef]
- Chen, S.R., et al., Ultrasound-guided perineural injection with dextrose for treatment of radial nerve palsy: A case report. Medicine (Baltimore), 2018. 97(23): p. e10978. [CrossRef]
- García de Cortázar, U., et al., Intraneural Platelet-Rich Plasma Injections for the Treatment of Radial Nerve Section: A Case Report. J Clin Med, 2018. 7(2).
- Schwartz, D.A., 32 - Tendon Transfers, in Fundamentals of Hand Therapy (Second Edition), C. Cooper, Editor. 2014, Mosby: St. Louis. p. 438–456.
- Buchanan, B.K., K. Maini, and M.A. Varacallo, Radial Nerve Entrapment, in StatPearls. 2025, StatPearls Publishing.
- Copyright © 2025, StatPearls Publishing LLC.: Treasure Island (FL).
- Crum, B.A. and J.A. Strommen, Peripheral nerve stimulation and monitoring during operative procedures. Muscle Nerve, 2007. 35(2): p. 159–70. [CrossRef]
- Chaney B, N.M. Axonotmesis. 2023; Available from: frhttps://www.ncbi.nlm.nih.gov/books/NBK562304/om:.
- Ditty, B.J., N.B. Omar, and C.J. Rozzelle, Chapter 24 - Surgery for Peripheral Nerve Trauma, in Nerves and Nerve Injuries, R.S. Tubbs, et al., Editors. 2015, Academic Press: San Diego. p. 373–381.
- Han, B.R., et al., Clinical Features of Wrist Drop Caused by Compressive Radial Neuropathy and Its Anatomical Considerations. J Korean Neurosurg Soc, 2014. 55(3): p. 148–151. [CrossRef]
- Bruno, F., et al., Application of diffusion tensor imaging (DTI) and MR-tractography in the evaluation of peripheral nerve tumours: state of the art and review of the literature. Acta Biomed, 2019. 90(5-s): p. 68–76. [CrossRef]
- Phansopkar, P., et al., Post-operative rehabilitation in a traumatic rare radial nerve palsy managed with tendon transfers: a case report. Pan Afr Med J, 2020. 36: p. 141. [CrossRef]
- Liu, X., et al., Research Progress of Low-Intensity Pulsed Ultrasound in the Repair of Peripheral Nerve Injury. Tissue Eng Part B Rev, 2023. 29(4): p. 414–428. [CrossRef]
- Kimbrough, D.A., K. Mehta, and R.D. Wissman, Case of the season: Saturday Night Palsy. Semin Roentgenol, 2013. 48(2): p. 108–10. [CrossRef]
- Chang, K.-V., W.-T. Wu, and L. Özçakar, Ultrasound imaging and guidance in peripheral nerve entrapment: hydrodissection highlighted. Pain Management, 2020. 10(2): p. 97–106. [CrossRef]
- Bumbasirevic, M., et al., Radial nerve palsy. EFORT Open Rev, 2016. 1(8): p. 286–294.
- Wu, Y.T., et al., Efficacy of 5% Dextrose Water Injection for Peripheral Entrapment Neuropathy: A Narrative Review. Int J Mol Sci, 2021. 22(22). [CrossRef]
- Wu, Y.T., et al., Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life (Basel), 2022. 12(6). [CrossRef]
- Wu, Y.T., et al., Nerve hydrodissection for carpal tunnel syndrome: A prospective, randomized, double-blind, controlled trial. Muscle Nerve, 2019. 59(2): p. 174–180. [CrossRef]
- Wu, Y.T., et al., Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol, 2018. 84(4): p. 601–610. [CrossRef]
- Wu, Y.T., et al., Six-month efficacy of perineural dextrose for carpal tunnel syndrome: A Prospective, randomized, double-blind, controlled trial. Mayo Clin Proc, 2017. 92(8): p. 1179–1189. [CrossRef]
- Tsui, B.C. and B. Kropelin, The electrophysiological effect of dextrose 5% in water on single-shot peripheral nerve stimulation. Anesth Analg, 2005. 100(6): p. 1837–9. [CrossRef]
- Reeves, K.D., et al., A Novel Somatic Treatment for Post-traumatic Stress Disorder: A Case Report of Hydrodissection of the Cervical Plexus Using 5% Dextrose. Cureus, 2022. 14(4): p. e23909. [CrossRef]
- Nasiri, A., F. Rezaei Motlagh, and M.A. Vafaei, Efficacy comparison between ultrasound-guided injections of 5% dextrose with corticosteroids in carpal tunnel syndrome patients. Neurol Res, 2023. 45(6): p. 554–563. [CrossRef]
- Shen, Y.P., et al., Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: A prospective randomized, single-blind, head-to-head comparative trial. J Tissue Eng Regen Med, 2019. 13(11): p. 2009–2017.
- Lam, K.H.S., et al., Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J Pain Res, 2020. 13: p. 1957–1968. [CrossRef]
- Babaei-Ghazani, A., et al., Ultrasound-guided 5% dextrose prolotherapy versus corticosteroid injection in carpal tunnel syndrome: a randomized, controlled clinical trial. Pain management, 2022. 12(6): p. 687–697. [CrossRef]
- Aghaei, S., et al., Local Injection of 5% Dextrose Versus Triamcinolone in Carpal Tunnel Syndrome: a Randomized Clinical Trial. SN comprehensive clinical medicine, 2021. 4(1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).