1. Introduction
Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), commonly referred to as long COVID or long COVID-19, refers to the persistence of symptoms and health issues in some patients long after the acute phase of COVID-19 infection has resolved (Nalbandian et al., 2021; Davis et al., 2023). This condition has emerged as a significant public health challenge due to its diverse and often debilitating symptoms that affect various bodily systems, including respiratory, cardiovascular, and neurological functions (Carfì et al., 2020; Greenhalgh et al., 2020).
However, the definition of this condition has not been well described yet. For example, the U.S. Centers for Disease Control and Prevention uses the term "PASC" to describe the wide range of health consequences that can persist for four weeks or more following SARS-CoV-2 infection (Centers for Disease Control and Prevention, 2024). Furthermore, the European Society of Clinical Microbiology and Infectious Diseases defines long COVID as the persistence or recurrence of one or more symptoms beyond 12 weeks after a confirmed COVID-19 infection, which cannot be explained by alternative diagnoses (Yelin et al., 2022). Similarly, the National Institute for Health and Care Excellence in the United Kingdom differentiates between ongoing symptomatic COVID-19, occurring from 4 to 12 weeks post-infection, and post-COVID-19 syndrome, which includes symptoms persisting beyond 12 weeks (National Institute for Health and Care Excellence, 2020). These definitions emphasize that long COVID is not a single disease but rather a condition with a broad spectrum of clinical manifestations (World Health Organization, 2021).
Identifying reliable diagnostic biomarkers for long COVID is crucial for early detection, patient stratification, and the development of personalized treatment strategies. Biomarkers, which are measurable indicators of biological states or conditions, can provide valuable insights into the underlying pathophysiological mechanisms of long COVID (Espín et al., 2023; Y.-J. Lai et al., 2023; Nalbandian et al., 2021). However, it’s not well known whether there is a specific biomarker for long COVID exists or which markers are associated with long COVID.
A bibliometric analysis involves the quantitative analysis of scientific publications to measure research output, influence, and collaboration patterns. This approach can help identify high-impact studies, leading researchers, significant research institutions, and most frequently used keywords, providing a detailed overview of the research dynamics (Guler et al., 2016; Ninkov et al., 2022; Pritchard, 1969).
This study aims to conduct a comprehensive bibliometric analysis of the literature to identify available biomarkers associated with long COVID and map the current research landscape, key trends, and research gaps.
2. Methods
2.1. Initial Data Collection
2.1.1. Data Source
A comprehensive bibliometric analysis of the literature on long COVID diagnostic biomarkers involves the quantitative analysis of scientific publications to measure research output, influence, and collaboration patterns. This approach can help identify high-impact studies, leading researchers, and significant research institutions, providing a detailed overview of the research dynamics (Ninkov et al., 2022; van Eck & Waltman, 2014). The Scopus database is exclusively used for literature search due to its comprehensive coverage of global academic information across multiple disciplines and its adherence to Bradford's law, making it particularly suitable for bibliometric analysis (Burnham, 2006).
2.1.2. Keyword Search
Conduct a literature search using the following specific keyword string:
(TITLE-ABS-KEY("Long COVID" OR "Post-acute Sequelae of SARS-CoV-2 infection" OR "Post-COVID-19 Syndrome" OR "PASC" OR "Chronic COVID" OR "COVID-19 long-term effects" OR "Long haul COVID" OR "Long-term COVID" OR "Long term effects of COVID") AND TITLE-ABS-KEY("Diagnostic markers" OR "Biomarkers" OR "Markers" OR "Indicators" OR "Diagnostic indicators" OR "Prognostic markers" OR "Prognostic indicators" OR "Clinical markers"))
The initial search yields 815 documents.
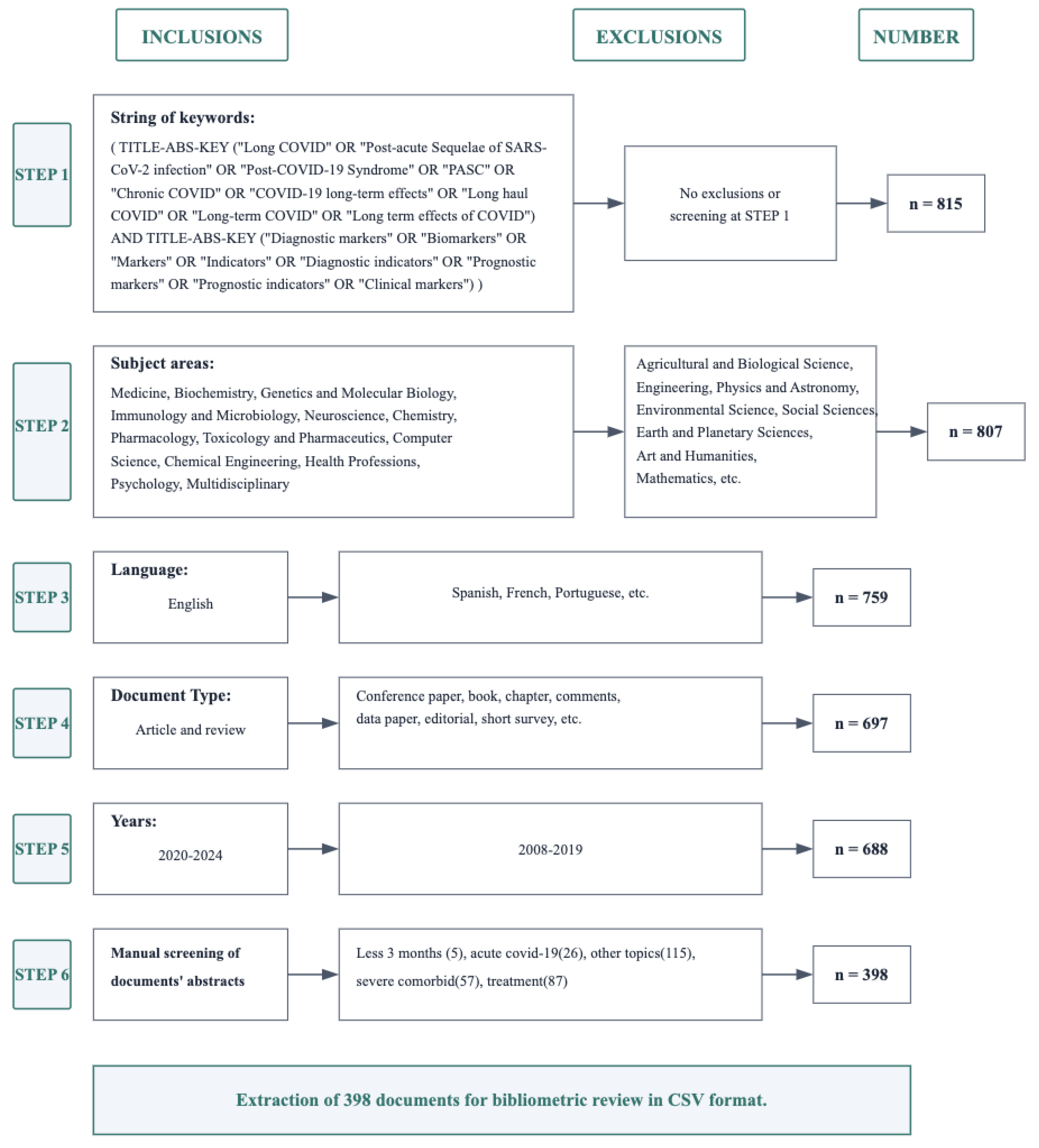
Figure 1.
Flow diagram of the literature search and selection process for Long COVID diagnostic biomarker studies. This flowchart illustrates the screening process starting from an initial set of 815 documents identified by the specified keywords (Step 1). Subsequent steps include selecting only documents from certain subject areas (Step 2), limiting language to English (Step 3), restricting document type (Step 4), and filtering by publication years (Step 5). Finally, a manual screening of abstracts (Step 6) was performed. A total of 398 documents fitting “Long COVID” and biomarker research requirements remained for the bibliometric analysis. In the figure, “n” indicates the number of documents remaining after each screening step.
Figure 1.
Flow diagram of the literature search and selection process for Long COVID diagnostic biomarker studies. This flowchart illustrates the screening process starting from an initial set of 815 documents identified by the specified keywords (Step 1). Subsequent steps include selecting only documents from certain subject areas (Step 2), limiting language to English (Step 3), restricting document type (Step 4), and filtering by publication years (Step 5). Finally, a manual screening of abstracts (Step 6) was performed. A total of 398 documents fitting “Long COVID” and biomarker research requirements remained for the bibliometric analysis. In the figure, “n” indicates the number of documents remaining after each screening step.
2.1.3. Literature Screening and Selection
A systematic screening process was implemented to identify relevant literature. Initial exclusion removed documents from non-relevant fields including Agricultural and Biological Sciences, Engineering, Physics and Astronomy, Environmental Science, Social Sciences, Earth and Planetary Sciences, Arts and Humanities, and Mathematics (n=807). Subsequently, non-English articles were excluded (n=759), followed by the removal of non-article and non-review types such as conference papers, book chapters, comments, data papers, editorials, and short surveys (n=697). The search was then restricted to publications from January 2020 to May 2024 (n=688). Finally, manual screening excluded studies focusing on acute COVID-19, treatments, other unrelated topics, and those with follow-up periods less than three months, yielding 398 articles for final analysis.
2.2. Data Extraction and Analysis
In this study, the R package "bibliometrix" was utilized for conducting bibliometric analysis. Additionally, "biblioshiny", an app providing a web interface for bibliometric analysis, was employed to extract variable data (Aria & Cuccurullo, 2017). We analyzed and visualized various aspects such as publication trends, journal distributions, author distributions, countries and institutions, collaborative networks, citation impact, and the most frequently cited papers globally, along with performing keyword clustering analysis. Journal impact factors and category information were based on the "2024 Journal Citation Reports" (JCR, 2024).
For visualization, bibliometrix (version 4.1.2) and VOSviewer (version 1.6.19) were used to map co-authorship and keyword co-occurrence among countries, institutions, and authors to enhance the understanding of long COVID research (Z. Lai et al., 2024; Yu et al., 2020). Biomarkers were selected based on frequency of citation (>5% of articles analyzed), clinical relevance in cited studies, and confirmed associations with persistent long COVID symptoms identified in high-impact literature (citation threshold: >50 citations).
3. Results
3.1. Document Types and Source Analysis
From January 2020 to May 2024, research on diagnostic biomarkers for long COVID has shown a significant increase (
Figure 2).
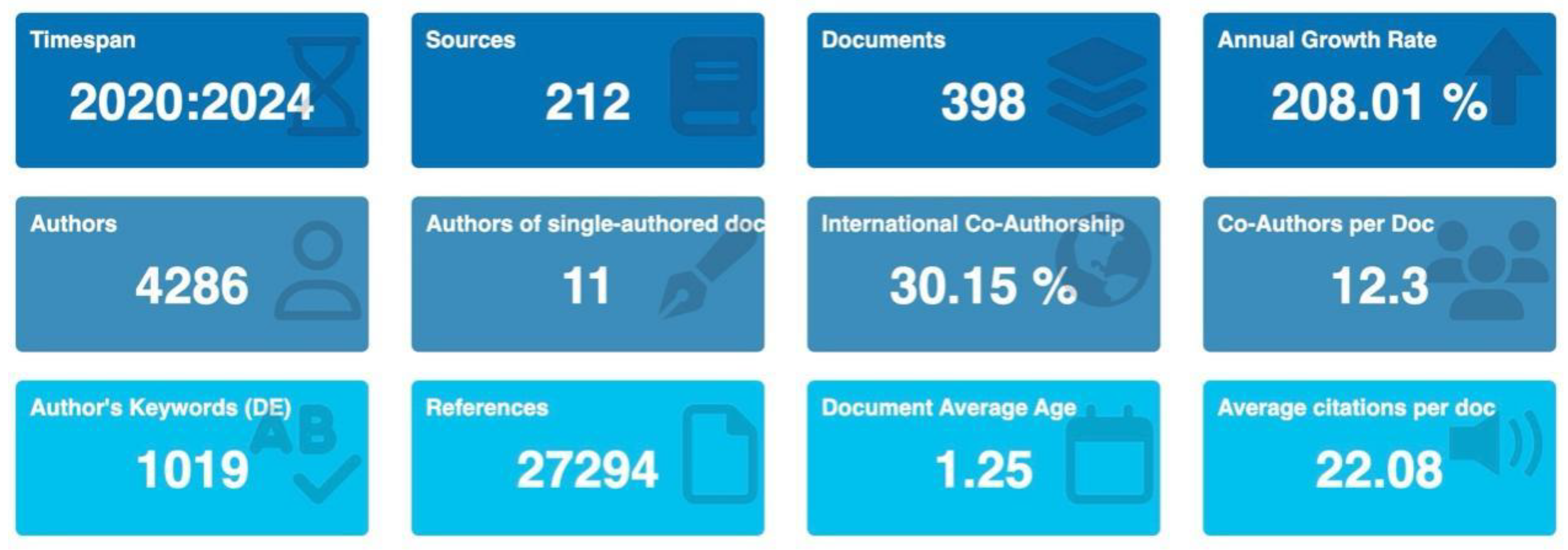
A total of 398 documents were published across 212 sources. The annual growth rate of publications is 208.01%, highlighting the rapidly growing interest and urgent need for understanding long COVID diagnostics.
As depicted in
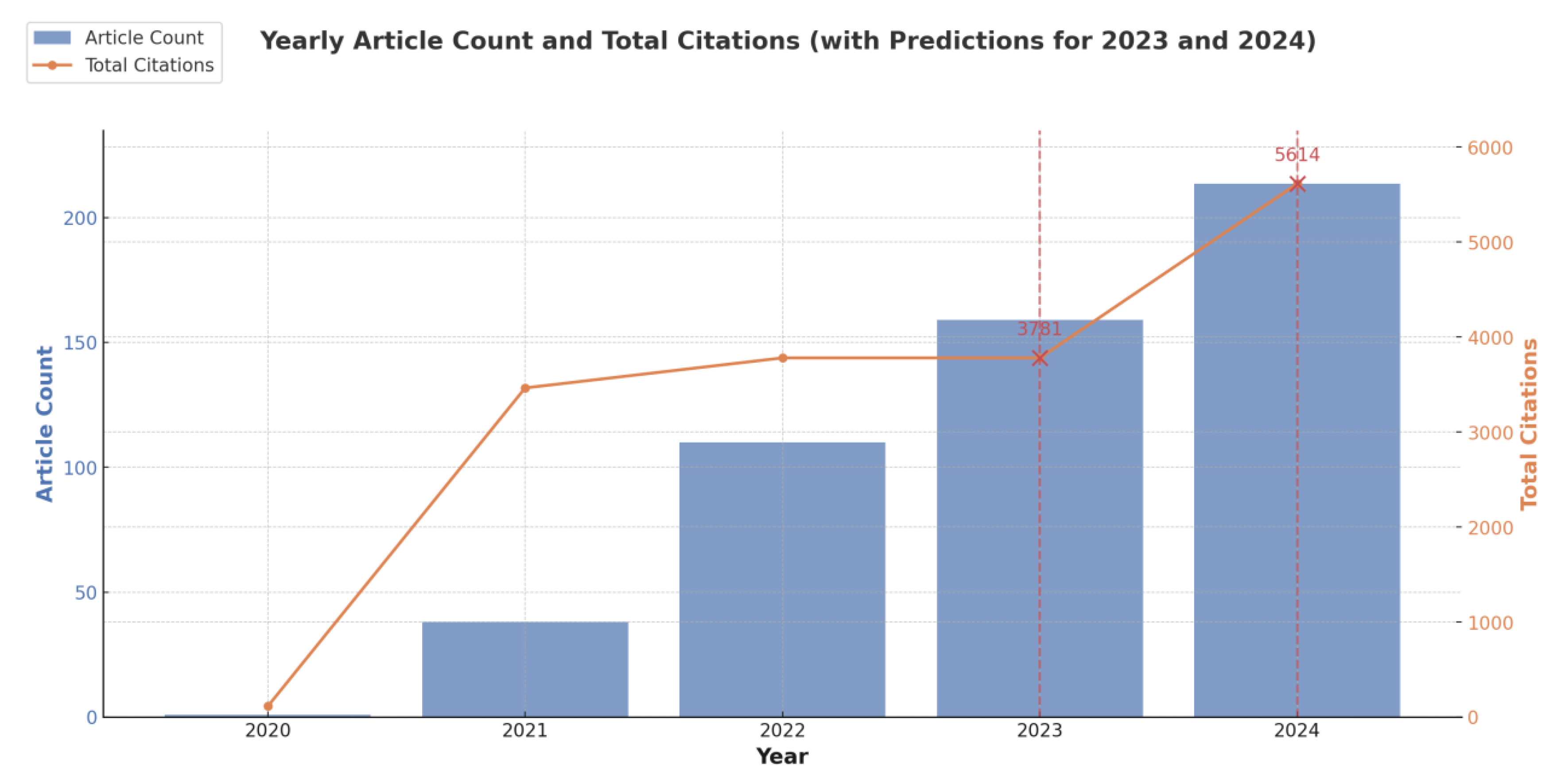
Figure 3, the number of articles published per year has increased steadily, with jump starting from 2021 (
Figure 3). In 2023, approximately 175 articles were published, and the predicted values for 2024 indicate a continued upward trend, with an estimated 200 articles. This suggests a sustained and growing interest in the field.
3.2. Author and Authorship Analysis
A total of 4,286 authors contributed to the body of work on long COVID biomarkers. The average number of co-authors per document is 12.3, reflecting a high level of collaboration within the research community. Only 11 documents were single authored, indicating a strong preference for collaborative research efforts. International co-authorship accounted for 30.15% of the publications, underscoring the global nature of research in this field (
Figure 2).
3.3. Document Age and Research Impact
The average age of the documents is 1.25 years, indicating the recent and rapidly evolving nature of research on long COVID biomarkers. This metric underscores the ongoing advancements and the up-to-date nature of the research outputs. Additionally, the high citation counts for key publications reflect the significant impact and influence of these studies in the scientific community (
Figure 2).
3.4. Geographic and Institutional Contributions
The United States emerged as the leading contributor, with 114 publications and significant contributions from institutions including Harvard University, which had 11 publications and 417 citations (
Table 1). Other notable contributors included Germany, Italy, and the United Kingdom, with Charité – Universitätsmedizin Berlin and University College London being key institutions in these countries.
3.5. Author Productivity and Impact
Author productivity and impact analysis revealed significant contributions from several key researchers in the field of long COVID diagnostic biomarkers.
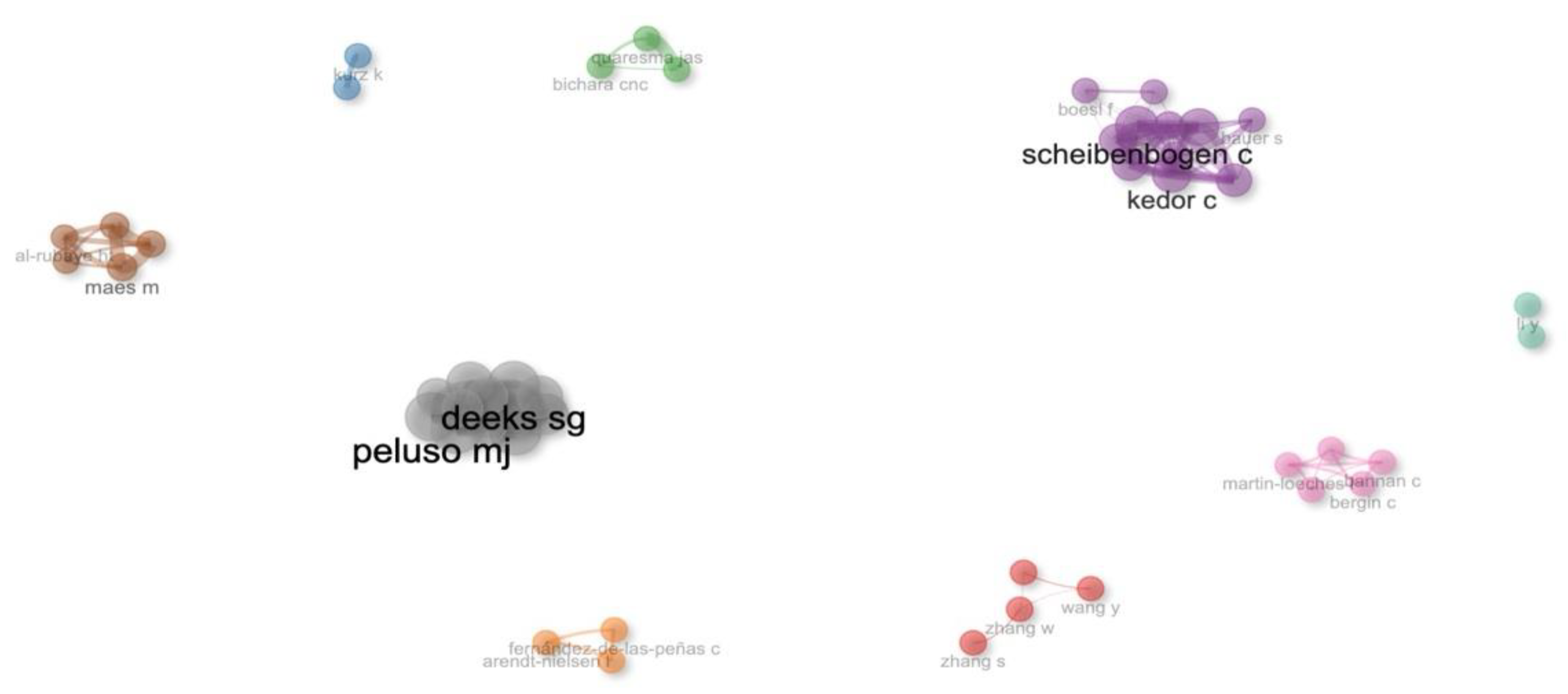
Figure 4 illustrates the relationship between the number of publications and the total citations received by individual authors, providing insight into both the quantity and influence of their research output. This visualization helps identify the most prolific and impactful researchers in the field. The analysis revealed that a total of 4,286 authors contributed to the body of work on long COVID biomarkers, with an average of 12.3 co-authors per document. Only 11 documents were single-authored, indicating a strong preference for collaborative research efforts. International co-authorship accounted for 30.15% of the publications, underscoring the global nature of research in this field. The most productive authors in this field included Peluso Michael J. from the University of California at San Francisco, and Scheibenbogen Carmen M. from Charité – Universitätsmedizin Berlin, each with 7 publications (
Table 2). These authors have significantly influenced the field, as evidenced by their high citation counts and h-indices. (
Figure 4)
3.6. Journal Analysis
The top journals publishing research on long COVID diagnostic biomarkers include "Frontiers in Immunology," "International Journal of Molecular Sciences," and "Journal of Clinical Medicine" (
Table 3). These journals have consistently published high-impact articles, with "Frontiers in Immunology" leading with 29 publications and a high citation score.
3.7. Highly Cited Articles
The article titled “Long COVID or Post-COVID-19 Syndrome: Putative Pathophysiology, Risk Factors, and Treatments” by Yong S.J. et al. (2021) stands out as the most cited, with an impressive 653 citations (
Table 4). Other highly cited works delve into the pathophysiology, risk factors, and long -term effects of COVID-19, underscoring the pivotal role these studies play in advancing understanding and driving progress in the field.
3.8. Keyword and Citation Analysis
The documents featured 1,019 unique keywords, suggesting a diverse range of research topics and approaches within the field. The total number of references cited across all documents is 27,294, indicating extensive academic dialogue and foundational research underpinning current studies. The average number of citations per document is 22.08, demonstrating the high impact and relevance of these studies within the scientific community (
Figure 2).
3.9. Network Analysis
The keyword co-occurrence network provided insights into the interconnectedness of various research themes. Central nodes such as C-reactive Protein(CRP), Interleukin-6(IL-6), and Tumor Necrosis Factor-alpha(TNF-α) indicate a predominant focus on inflammatory processes (
Figure 5 and
Figure 6). The network also highlighted significant connections to clinical symptoms like fatigue, dyspnea, and myalgia, emphasizing the multifaceted nature of long COVID and the need for a comprehensive diagnostic approach.
3.10. Collaborative Networks
The co-authorship network map revealed strong collaborative ties among researchers and institutions across different countries (
Figure 7). Major collaborative hubs were identified in the United States and Europe, with extensive links to Asia and Australia. This global collaboration is essential for addressing the complex and multifaceted challenges of long COVID.
3.11. Analysis of the Ten Most Representative Studies in the Field of Long COVID from 2020.1 to 2024.5
The ten most representative studies in long COVID biomarker research were selected based on rigorous criteria emphasizing both scientific impact and methodological quality. These studies were chosen from the highest-cited original research articles (excluding reviews and meta-analyses) within our dataset, with impact factors ranging from 5.5 to 54.4, ensuring inclusion of only the most influential clinical investigations. The selection prioritized prospective and cross-sectional studies that provided robust evidence for biomarker associations with long COVID symptoms, with sample sizes ranging from 42 to 384 patients and follow-up periods extending from median 54 days to over 8 months post-infection.
Table 5 was constructed to systematically present the clinical characteristics and biomarker profiles from these ten pivotal studies, enabling direct comparison of findings across different research groups and methodologies. The table consolidates critical information including study design, sample demographics, symptom prevalence, and specific biomarker measurements, providing a comprehensive overview of the current evidence base.
Analysis of
Table 5 reveals consistent patterns across multiple independent investigations. The most frequently reported clinical manifestations include fatigue (ranging from 51% to 100% across studies), cognitive impairment and brain fog (49% to 87%), and respiratory symptoms including breathlessness and persistent cough (34% to 80%). Regarding biomarker findings, inflammatory markers demonstrated persistent elevation, with interleukin-6, tumor necrosis factor-alpha, and C-reactive protein showing sustained abnormalities months after acute infection. Cardiovascular biomarkers including D-dimer, troponin, and natriuretic peptides indicated ongoing cardiac and vascular dysfunction. Neurological markers, particularly neurofilament light chain and glial fibrillary acidic protein, correlated with cognitive symptoms and sensory disturbances.
Based on the convergent findings from
Table 5 and the broader literature analysis, we established a four-system biomarker framework that captures the multisystem pathophysiology of long COVID. This categorization was developed because the identified biomarkers clustered into distinct pathophysiological domains: immune-inflammatory, cardiovascular, neurological, and metabolic systems. This systematic approach provides a structured framework for understanding the complex, interconnected nature of long COVID pathophysiology while facilitating clinical translation and future research directions.
Beyond the core findings presented in
Table 5, comprehensive analysis of the broader literature (detailed in
Supplementary Materials 2) reveals an extensive array of biomarkers spanning multiple biological systems. The immune-inflammatory system demonstrates the most extensive dysregulation, with elevated levels of numerous cytokines, chemokines, and acute-phase proteins. Complement system activation markers, including C1s-C1 inhibitor complex and terminal complement complex, indicate persistent immune activation. Adaptive immune dysfunction manifests through altered T-cell populations and autoantibody production against various self-antigens.
The four-system biomarker framework encompasses distinct but interconnected pathophysiological domains. The immune-inflammatory system, characterized by persistent elevation of IL-6, TNF-α, and CRP, correlates with fatigue, persistent cough, and depressive symptoms. Research by Peluso et al. and Phetsouphanh et al. demonstrates that these inflammatory mediators remain elevated months after acute infection, suggesting fundamental disruption of immune resolution processes.
Cardiovascular system biomarkers, including troponin, NT-proBNP, and D-dimer, reflect ongoing cardiac injury and endothelial dysfunction. Studies by Mandal et al. and Townsend et al. document persistent elevation of these markers correlating with breathlessness, chest pain, and exercise intolerance. The work of Fogarty et al. specifically demonstrates endothelial activation through von Willebrand factor and thrombomodulin elevation, indicating persistent vascular dysfunction.
Neurological system involvement is evidenced through neurofilament light chain and glial fibrillary acidic protein abnormalities, markers that directly correlate with brain fog, cognitive impairment, and sensory symptoms including anosmia and ageusia. Research by Klein et al. and imaging studies by Guedj et al. provide convergent evidence for widespread nervous system involvement extending beyond the acute phase of infection.
Metabolic system dysregulation manifests through insulin resistance markers (HOMA2-IR) and lipid metabolism abnormalities, reflecting cellular energy metabolism disruption. These findings, combined with mitochondrial dysfunction markers, suggest fundamental alterations in cellular bioenergetics that may underlie the persistent fatigue and exercise intolerance characteristic of long COVID.
4. Discussion
The findings suggest that although no specific biomarkers currently exist for long COVID diagnosis, there is strong evidence that dysregulation occurs in multiple biological systems, including immune, cardiovascular, neurological, and metabolic regulations (
Figure 8).
Keyword co-occurrence network analysis reveals the centrality of inflammatory markers in long COVID research and their close association with multi-system symptoms. This multi-system biomarker pattern reflects the complex pathophysiology of long COVID, involving interactions across immune, neurological, metabolic, and cardiovascular systems (Nalbandian et al., 2021; Ceban et al., 2022).
The most consistently reported biomarkers in long COVID are inflammatory mediators, particularly IL-6, TNF-α, and CRP. These markers are closely associated with fatigue, persistent cough, and depressive symptoms (Peluso & Deeks, 2024). Their persistent elevation beyond the acute phase indicates fundamental disruption of immune resolution processes, establishing a pathological cycle where initial tissue damage promotes ongoing inflammation (Davis et al., 2023; Lai et al., 2023).
At the molecular level, IL-6 activates the JAK-STAT signaling pathway affecting hypothalamic-pituitary-adrenal axis function, potentially providing a biochemical basis for fatigue symptoms. Similarly, sustained TNF-α elevation correlates with hippocampal neurogenesis inhibition and decreased neuroplasticity, offering a neurobiological explanation for depressive symptoms (Almulla et al., 2024; Arish et al., 2023). The production of autoantibodies, such as antinuclear antibodies, may explain symptom persistence after viral clearance through "molecular mimicry" mechanisms (Chang et al., 2021; Son et al., 2023).
Neurological biomarkers including neurofilament light chain and glial fibrillary acidic protein directly reflect neuronal damage and astrocytic activation, correlating with brain fog, cognitive impairment, and anosmia/ageusia (Bark et al., 2023; Plantone et al., 2024). Abnormal brain metabolism patterns on FDG-PET imaging further corroborate these findings (Guedj et al., 2021; Gutman et al., 2024).
High-sensitivity troponin, NT-proBNP, and D-dimer as cardiovascular biomarkers reflect myocardial injury and endothelial dysfunction, associated with breathlessness, chest pain, and exercise intolerance (Aboughdir et al., 2020; Yaluri et al., 2023). Endothelial cells become direct targets for SARS-CoV-2 via ACE2 receptors, leading to microvascular dysfunction and impaired tissue perfusion (Lindner et al., 2020; Tavazzi et al., 2020). This "endotheliopathy" provides a unifying framework for multi-organ involvement, as microcirculatory dysfunction simultaneously affects cardiac, pulmonary, and cerebral tissues (Bellone et al., 2024; van den Berg et al., 2023). Elevated endothelial activation markers such as von Willebrand factor further confirm this mechanism (Ackermann et al., 2020; Tang et al., 2020).
These neurological biomarkers are closely linked to microglial activation and neuroinflammatory responses, particularly in cognitive-critical regions including the hippocampus, prefrontal cortex, and insula (Díez-Cirarda et al., 2023; Frontera et al., 2022). Notably, these patterns share similarities with those observed in certain neurodegenerative disorders, suggesting long COVID may share common neural injury mechanisms (Douaud et al., 2022; Rogers et al., 2020).
Metabolic dysregulation biomarkers HOMA2-IR and triglycerides reflect cellular energy metabolism disruption in long COVID patients, associated with glucose and lipid metabolism abnormalities (Al-Hakeim et al., 2023; Szögi et al., 2024). Mitochondrial dysfunction represents a core mechanism, manifesting as reduced ATP production, altered membrane potential, and increased oxidative stress (Ayola-Serrano et al., 2021).
Metabolomic studies further reveal alterations in tricarboxylic acid cycle intermediates and lipid peroxidation products, patterns that significantly overlap with those reported in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (Fernández-Lázaro et al., 2021; Haque & Pant, 2022), explaining the prevalent fatigue and post-exertional malaise in long COVID.
Autonomic nervous system dysfunction is common in long COVID patients, associated with Postural Orthostatic Tachycardia Syndrome (POTS) and other orthostatic symptoms. Biomarkers including altered catecholamine levels, reduced heart rate variability, and abnormal vasomotor responses reflect sympathetic-parasympathetic imbalance (Akbarialiabad et al., 2021; Phetsouphanh et al., 2022).
This autonomic dysfunction interacts with endothelial dysregulation, explaining orthostatic intolerance, dizziness, and palpitations (Su et al., 2022; K. Yin et al., 2024). Autonomic dysregulation may also represent a common mechanism underlying multiple long COVID symptoms.
Coagulation abnormalities constitute another key feature of long COVID, with alterations in D-dimer, fibrinogen, and platelet factor 4 reflecting a persistent hypercoagulable state (Gameil et al., 2021; Mohd Zawawi et al., 2023). This coagulation imbalance may lead to microthrombi formation, further exacerbating tissue hypoxia and organ dysfunction.
Complex interactions exist between coagulation and inflammatory responses, forming an "immunothrombosis" phenomenon (Sollini et al., 2021; Son et al., 2023). This coagulation-inflammation interplay explains the coexistence of multiple symptoms including fatigue, dyspnea, and cognitive impairment (Bellone et al., 2024; Guedj et al., 2021).
5. Limitations
This bibliometric analysis is subject to several limitations. First, our investigation was confined to articles indexed in the Scopus database, potentially excluding pertinent publications—especially those in non-English languages or in journals not covered by Scopus (Falagas et al., 2008; Mongeon & Paul-Hus, 2015). Second, given the rapidly evolving nature of long COVID research, with continuous updates and an influx of new publications, our analysis represents only a snapshot of the research landscape as of the search date. Third, although bibliometric analysis provides valuable quantitative insights into research trends and collaborative networks, it does not capture qualitative dimensions, such as the clinical significance of the findings or the methodological rigor of the study designs. Our analysis, while comprehensive, relies on bibliometric data limited to published and highly cited literature, potentially overlooking emerging biomarkers or those only recently gaining attention. Future reviews could overcome these limitations by integrating bibliometric methods with systematic review approaches, thereby offering a more comprehensive appraisal of biomarker research in long COVID. Moreover, expanding the scope to include additional databases and employing more advanced analytical techniques may further elucidate the evolving research landscape in this field (Davis et al., 2023; Espín et al., 2023; Z. Lai et al., 2024). Future primary research studies should validate biomarkers identified here, especially those showing strong theoretical support but limited current citation impact.
6. Conclusion
Based on our systematic bibliometric analysis, we found that although no specific biomarkers currently exist for definitive long COVID diagnosis, there is compelling evidence of sustained dysregulation across multiple biological systems. Notably, the persistent elevation of inflammatory markers (including IL-6, TNF-α, and CRP), together with abnormalities in cardiovascular and neurological injury biomarkers, suggests a complex pathophysiological process involving multiple organ systems. These findings advance our understanding of long COVID's underlying mechanisms and may inform future clinical investigations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
NS: Methodology, Writing (review and editing), Supervision. XL: Data Acquisition, Data Curation, Statistical Analysis, Visualization, Literature Search, Writing (original draft and editing). CX: Data Curation, Acquisition, Visualization. TS: Writing (review and editing). BL: Methodology, Writing (review and editing).
Funding
This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (JP22K07480).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. (2021). Post-acute COVID-19 syndrome. Nature Medicine, 27, 601–615. [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. (2023). Long COVID: Major findings, mechanisms and recommendations. Nature Reviews Microbiology, 21, 133–146. [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. (2020). Persistent symptoms in patients after acute COVID-19. JAMA, 324, 603–605. [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. (2020). Management of post-acute covid-19 in primary care. BMJ, 370, m3026. [CrossRef]
- Centers for Disease Control and Prevention[CDC]. (2024, November 13). Clinical overview of long COVID. Covid-19. Available online: https://www.cdc.gov/covid/hcp/clinical-overview/index.html.
- Yelin, D.; Moschopoulos, C.D.; Margalit, I.; Gkrania-Klotsas, E.; Landi, F.; Stahl, J.-P.; Yahav, D. (2022). ESCMID rapid guidelines for assessment and management of long COVID. Clinical Microbiology and Infection, 28, 955–972. [CrossRef]
- National Institute for Health and Care Excellence [NICE]. (2020, December 18). Overview | COVID-19 rapid guideline: Managing the long -term effects of COVID-19 | guidance | NICE. NICE. Available online: https://www.nice.org.uk/guidance/ng188.
- World Health Organization. (2021, October 6). A clinical case definition of post COVID-19 condition by a delphi consensus, 6 october 2021_case_definition-2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. (2023). Cellular and molecular biomarkers of long COVID: A scoping review. eBioMedicine, 91. [CrossRef]
- Lai, Y.-J.; Liu, S.-H.; Manachevakul, S.; Lee, T.-A.; Kuo, C.-T.; Bello, D. (2023). Biomarkers in long COVID-19: A systematic review. Frontiers in Medicine, 10, 1085988. [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. (2021). Symptoms, complications and management of long COVID: A review. Journal of the Royal Society of Medicine, 114, 428–442. [CrossRef]
- Guler, A.T.; Waaijer, C.J.F.; Palmblad, M. (2016). Scientific workflows for bibliometrics. Scientometrics, 107, 385–398. [CrossRef]
- Ninkov, A.; Frank, J.R.; Maggio, L.A. (2022). Bibliometrics: Methods for studying academic publishing. Perspectives on Medical Education, 11, 173–176. [CrossRef]
- Pritchard, A. (1969). Statistical bibliography or bibliometrics. Journal of Documentation. Available online: https://www.semanticscholar.org/paper/Statistical-bibliography-or-bibliometrics-Pritchard/0be426317b9001813ece55e91c77281e9bd48205.
- van Eck, N.J.; Waltman, L. (2014). Visualizing bibliometric networks. In Y. Ding, R. Rousseau, & D. Wolfram (Eds.), Measuring Scholarly Impact: Methods and Practice (pp. 285–320). Springer International Publishing. [CrossRef]
- Burnham, J.F. (2006). Scopus database: A review. Biomedical Digital Libraries, 3, 1. [CrossRef]
- Aria, M.; Cuccurullo, C. (2017). bibliometrix: An R-tool for comprehensive science mapping analysis. Journal of Informetrics, 11, 959–975. [CrossRef]
- JCR. (2024, November 1). Journal Citation Reports. Available online: https://journalcitationreports.zendesk.com/hc/en-gb/articles/28351055638801-2024.
- Lai, Z.; Pu, T.; Li, J.; Bai, F.; Wu, L.; Tang, Y. (2024). Visual analysis of hotspots and trends in long COVID research based on bibliometric. Heliyon, 10. [CrossRef]
- Yu, Y.; Li, Y.; Zhang, Z.; Gu, Z.; Zhong, H.; Zha, Q.; Yang, L.; Zhu, C.; Chen, E. (2020). A bibliometric analysis using VOSviewer of publications on COVID-19. Annals of Translational Medicine, 8, 816. [CrossRef]
- Bohmwald, K.; Diethelm-Varela, B.; Rodríguez-Guilarte, L.; Rivera, T.; Riedel, C.A.; González, P.A.; Kalergis, A.M. (2024). Pathophysiological, immunological, and inflammatory features of long COVID. Frontiers in Immunology, 15. [CrossRef]
- Mateu, L.; Tebe, C.; Loste, C.; Santos, J.R.; Lladós, G.; López, C.; España-Cueto, S.; Toledo, R.; Font, M.; Chamorro, A.; et al. (2023). Determinants of the onset and prognosis of the post-COVID-19 condition: A 2-year prospective observational cohort study. The Lancet Regional Health – Europe, 33. [CrossRef]
- Rathod, N.; Kumar, S.; Chavhan, R.; Acharya, S.; Rathod, S. (2024). Navigating the long haul: A comprehensive review of long -COVID sequelae, patient impact, pathogenesis, and management. Cureus. [CrossRef]
- Liew, F.; Efstathiou, C.; Fontanella, S.; Richardson, M.; Saunders, R.; Swieboda, D.; Sidhu, J.K.; Ascough, S.; Moore, S.C.; Mohamed, N.; et al. (2024). Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nature Immunology, 25, 607–621. [CrossRef]
- Luan, Y.-Y.; Yin, C.-H.; Yao, Y.-M. (2021). Update advances on C-reactive protein in COVID-19 and other viral infections. Frontiers in Immunology, 12, 720363. [CrossRef]
- Yin, J.-X.; Agbana, Y.L.; Sun, Z.-S.; Fei, S.-W.; Zhao, H.-Q.; Zhou, X.-N.; Chen, J.-H.; Kassegne, K. (2023). Increased interleukin-6 is associated with long COVID-19: A systematic review and meta-analysis. Infectious Diseases of Poverty, 12, 43. [CrossRef]
- Almulla, A.F.; Thipakorn, Y.; Zhou, B.; Vojdani, A.; Maes, M. (2024). Immune activation and immune-associated neurotoxicity in long -COVID: A systematic review and meta-analysis of 103 studies comprising 58 cytokines/chemokines/growth factors. Brain, Behavior and Immunity, 122, 75–94. [CrossRef]
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. (2023). COVID-19 immunopathology: From acute diseases to chronic sequelae. Journal of Medical Virology, 95, e28122. [CrossRef]
- Gomes, S.M.R.; Brito, A.C. de S.; Manfro, W.F.P.; Ribeiro-Alves, M.; Ribeiro, R.S. de A.; da Cal, M.S.; Lisboa, V. da C.; Abreu, D.P.B. de, Castilho, L.D.R.; Porto, L.C.; et al. (2023). High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long -COVID-19 patients. PLOS One, 18, e0283983. [CrossRef]
- Gusev, E.; Sarapultsev, A. (2024). Exploring the pathophysiology of long COVID: The central role of low-grade inflammation and multisystem involvement. International Journal of Molecular Sciences, 25, Article 12. [CrossRef]
- Fernández-Lázaro, D.; Sánchez-Serrano, N.; Mielgo-Ayuso, J.; García-Hernández, J.L.; González-Bernal, J.J.; Seco-Calvo, J. (2021). Long COVID a new derivative in the chaos of SARS-CoV-2 infection: The emergent pandemic? Journal of Clinical Medicine, 10, Article 24. [CrossRef]
- Haque, A.; Pant, A.B. (2022). Mitigating covid-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. Journal of Autoimmunity, 127, 102792. [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. (2024). The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomedicine & Pharmacotherapy, 178, 117177. [CrossRef]
- Song, Y.; Li, J.; Wu, Y. (2024). Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders. Signal Transduction and Targeted Therapy, 9, 1–40. [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. (2023). Immunomodulation—A general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Frontiers in Immunology, 14. [CrossRef]
- Bark, L.; Larsson, I.-M.; Wallin, E.; Simrén, J.; Zetterberg, H.; Lipcsey, M.; Frithiof, R.; Rostami, E.; Hultström, M. (2023). Central nervous system biomarkers GFAp and NfL associate with post-acute cognitive impairment and fatigue following critical COVID-19. Scientific Reports, 13, 13144. [CrossRef]
- Huang, Z.; Haile, K.; Gedefaw, L.; Lau, B.W.M.; Jin, L.; Yip, S.P.; Huang, C.L. (2023). Blood biomarkers as prognostic indicators for neurological injury in COVID-19 patients: A systematic review and meta-analysis. International Journal of Molecular Sciences, 24. [CrossRef]
- Plantone, D.; Stufano, A.; Righi, D.; Locci, S.; Iavicoli, I.; Lovreglio, P.; De Stefano, N. (2024). Neurofilament light chain and glial fibrillary acid protein levels are elevated in post-mild COVID-19 or asymptomatic SARS-CoV-2 cases. Scientific Reports, 14, 6429. [CrossRef]
- Telser, J.; Grossmann, K.; Weideli, O.C.; Hillmann, D.; Aeschbacher, S.; Wohlwend, N.; Velez, L.; Kuhle, J.; Maleska, A.; Benkert, P.; et al. (2023). Concentrations of serum brain injury biomarkers following SARS-CoV-2 infection in individuals with and without long -COVID-results from the prospective population-based COVI-GAPP study. Diagnostics (Basel, Switzerland), 13, 2167. [CrossRef]
- Díez-Cirarda, M.; Yus-Fuertes, M.; Sanchez-Sanchez, R.; Gonzalez-Rosa, J.J.; Gonzalez-Escamilla, G.; Gil-Martínez, L.; Delgado-Alonso, C.; Gil-Moreno, M.J.; Valles-Salgado, M.; Cano-Cano, F.; et al. (2023). Hippocampal subfield abnormalities and biomarkers of pathologic brain changes: From SARS-CoV-2 acute infection to post-COVID syndrome. Ebiomedicine, 94. [CrossRef]
- Frontera, J.A.; Boutajangout, A.; Masurkar, A.V.; Betensky, R.A.; Ge, Y.; Vedvyas, A.; Debure, L.; Moreira, A.; Lewis, A.; Huang, J.; et al. (2022). Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or alzheimer’s dementia. Alzheimer’s & Dementia, 18, 899–910. [CrossRef]
- Hanson, B.A.; Visvabharathy, L.; Ali, S.T.; Kang, A.K.; Patel, T.R.; Clark, J.R.; Lim, P.H.; Orban, Z.S.; Hwang, S.S.; Mattoon, D.; et al. (2022). Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with postacute sequelae of SARS-CoV-2 infection. Neurology(R) Neuroimmunology & Neuroinflammation, 9, e1151. [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet. Psychiatry, 7, 611–627. [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. (2022). SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature, 604, 697–707. [CrossRef]
- Aboughdir, M.; Kirwin, T.; Abdul Khader, A.; Wang, B. (2020). Prognostic value of cardiovascular biomarkers in COVID-19: A review. Viruses, 12, Article 5. [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. (2020). Long itudinal analyses reveal immunological misfiring in severe COVID-19. Nature, 584, 463–469. [CrossRef]
- Samprathi, M.; Jayashree, M. (2021). Biomarkers in COVID-19: An up-to-date review. Frontiers in Pediatrics, 8. [CrossRef]
- Yaluri, N.; Stančáková Yaluri, A.; Žeňuch, P.; Žeňuchová, Z.; Tóth, Š.; Kalanin, P. (2023). Cardiac biomarkers and their role in identifying increased risk of cardiovascular complications in COVID-19 patients. Diagnostics, 13, Article 15. [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. (2020). Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiology, 5, 1281–1285. [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. (2020). Myocardial localization of coronavirus in COVID-19 cardiogenic shock. European Journal of Heart Failure, 22, 911–915. [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. New England Journal of Medicine, 383, 120–128. [CrossRef]
- Tang, N.; Bai, H.; Xiong, D.; Sun, Z. (2020). Specific coagulation markers may provide more therapeutic targets in COVID-19 patients receiving prophylactic anticoagulant. Journal of Thrombosis and Haemostasis, 18, 2428–2430. [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England), 395, 1417–1418. [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. (2022). Long -term cardiovascular outcomes of COVID-19. Nature Medicine, 28, 583–590. [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. (2021). Long COVID, a comprehensive systematic scoping review. Infection, 49, 1163–1186. [CrossRef]
- Gheorghita, R.; Soldanescu, I.; Lobiuc, A.; Caliman Sturdza, O.A.; Filip, R.; Constantinescu – Bercu, A.; Dimian, M.; Mangul, S.; Covasa, M. (2024). The knowns and unknowns of long COVID-19: From mechanisms to therapeutical approaches. Frontiers in Immunology, 15. [CrossRef]
- Qi, P.; Huang, M.; Zhu, H. (2023). Exploring potential biomarkers and therapeutic targets of long COVID-associated inflammatory cardiomyopathy. Frontiers in Medicine, 10, 1191354. [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. (2021). Post-COVID-19 symptom burden: What is long -COVID and how should we manage it? Lung, 199, 113–119. [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. (2022). Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nature Immunology, 23, 210–216. [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. (2022). Multiple early factors anticipate post-acute COVID-19 sequelae. Cell, 185, 881-895.e20. [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. (2024). Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nature Immunology, 25, 218–225. [CrossRef]
- Skevaki, C.; Moschopoulos, C.D.; Fragkou, P.C.; Grote, K.; Schieffer, E.; Schieffer, B. (2024). Long COVID: Pathophysiology, current concepts, and future directions. Journal of Allergy and Clinical Immunology, S0091-67492406-0. [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. (2021). The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduction and Targeted Therapy, 6, 1–20. [CrossRef]
- Gameil, M.A.; Marzouk, R.E.; Elsebaie, A.H.; Rozaik, S.E. (2021). Long -term clinical and biochemical residue after COVID-19 recovery. Egyptian Liver Journal, 11, 74. [CrossRef]
- Mohd Zawawi, Z.; Kalyanasundram, J.; Mohd Zain, R.; Thayan, R.; Basri, D.F.; Yap, W.B. (2023). Prospective roles of tumor necrosis factor-alpha (TNF-α) in COVID-19: Prognosis, therapeutic and management. International Journal of Molecular Sciences, 24, 6142. [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. (2021). New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nature Communications, 12, 5417. [CrossRef]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. (2021). Long COVID hallmarks on [18F]FDG-PET/CT: A case-control study. European Journal of Nuclear Medicine and Molecular Imaging, 48, 3187–3197. [CrossRef]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. (2023). Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. European Respiratory Journal, 61. [CrossRef]
- Bellone, S.; Siegel, E.R.; Scheim, D.E.; Santin, A.D. (2024). Increased von willebrand and factor VIII plasma levels in gynecologic patients with post-acute-COVID-sequela (PASC)/long COVID. Gynecologic Oncology Reports, 51, 101324. [CrossRef]
- van den Berg, J.; Haslbauer, J.D.; Stalder, A.K.; Romanens, A.; Mertz, K.D.; Studt, J.-D.; Siegemund, M.; Buser, A.; Holbro, A.; Tzankov, A. (2023). Von willebrand factor and the thrombophilia of severe COVID-19: In situ evidence from autopsies. Research and Practice in Thrombosis and Haemostasis, 7, 100182. [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. (2021). 18F-FDG brain PET hypometabolism in patients with long COVID. European Journal of Nuclear Medicine and Molecular Imaging, 48, 2823–2833. Scopus. [CrossRef]
- Gutman, E.G.; Salvio, A.L.; Fernandes, R.A.; Duarte, L.A.; Raposo-Vedovi, J.V.; Alcaraz, H.F.; Teixeira, M.A.; Passos, G.F.; de Medeiros, K.Q.M.; Hammerle, M.B.; et al. (2024). Long COVID: Plasma levels of neurofilament light chain in mild COVID-19 patients with neurocognitive symptoms. Molecular Psychiatry, 29, 3106–3116. Scopus. [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. (2023). Increased insulin resistance due to long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Brazilian Journal of Psychiatry, 45, 205–215. Scopus. [CrossRef]
- Ayola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S.; et al. (2021). The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflammation Research, 70, 877–889. [CrossRef]
- Szögi, T.; Borsos, B.N.; Masic, D.; Radics, B.; Bella, Z.; Bánfi, A.; Ördög, N.; Zsiros, C.; Kiricsi, Á.; Pankotai-Bodó, G.; et al. (2024). Novel biomarkers of mitochondrial dysfunction in long COVID patients. Geroscience. [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. (2022). Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain, Behavior and Immunity, 101, 93–135. [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. (2023). Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 76, e487–e490. [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. (2022). A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infectious Diseases, 22, e102–e107. [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; Molteni, E.; Modat, M.; et al. (2021). Attributes and predictors of long COVID. Nature Medicine, 27, 626–631. [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. (2021). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. The Lancet. Psychiatry, 8, 416–427. [CrossRef]
Figure 2.
Annual publication trend of Long COVID diagnostic biomarker research from 2020 to 2024. This figure shows key bibliometric indicators for 398 documents published from 2020 to May 9, 2024, spanning 212 sources and an annual growth rate of 208.01%. A total of 4,286 authors contributed (including 11 single-authored papers), with 30.15% of publications involving international co-authorship and an average of 12.3 co-authors per paper. Overall, 1,019 unique keywords were used, referencing 27,294 sources. On average, each document is 1.25 years old and has been cited 22.08 times.
Figure 2.
Annual publication trend of Long COVID diagnostic biomarker research from 2020 to 2024. This figure shows key bibliometric indicators for 398 documents published from 2020 to May 9, 2024, spanning 212 sources and an annual growth rate of 208.01%. A total of 4,286 authors contributed (including 11 single-authored papers), with 30.15% of publications involving international co-authorship and an average of 12.3 co-authors per paper. Overall, 1,019 unique keywords were used, referencing 27,294 sources. On average, each document is 1.25 years old and has been cited 22.08 times.
Figure 3.
Predicted publication trend for Long COVID diagnostic biomarker research up to 2024. This bar chart illustrates the annual article count (blue bars, left axis) from 2020 through 2024 along side total citations (orange line, right axis). Because data for 2023 and 2024 were still incomplete when collected, their totals (indicated with dashed vertical lines and “X” markers) reflect predicted values rather than final counts.
Figure 3.
Predicted publication trend for Long COVID diagnostic biomarker research up to 2024. This bar chart illustrates the annual article count (blue bars, left axis) from 2020 through 2024 along side total citations (orange line, right axis). Because data for 2023 and 2024 were still incomplete when collected, their totals (indicated with dashed vertical lines and “X” markers) reflect predicted values rather than final counts.
Figure 4.
Author productivity and impact in Long COVID diagnostic biomarker research. This network visualization illustrates co-authorship clusters among researchers in the field of Long COVID diagnostic biomarker research. Each color-coded node represents an author, with larger nodes indicating higher productivity or citation impact. The proximity of the nodes reflects how often the authors collaborate, while larger labels denote authors with greater influence in the network.
Figure 4.
Author productivity and impact in Long COVID diagnostic biomarker research. This network visualization illustrates co-authorship clusters among researchers in the field of Long COVID diagnostic biomarker research. Each color-coded node represents an author, with larger nodes indicating higher productivity or citation impact. The proximity of the nodes reflects how often the authors collaborate, while larger labels denote authors with greater influence in the network.
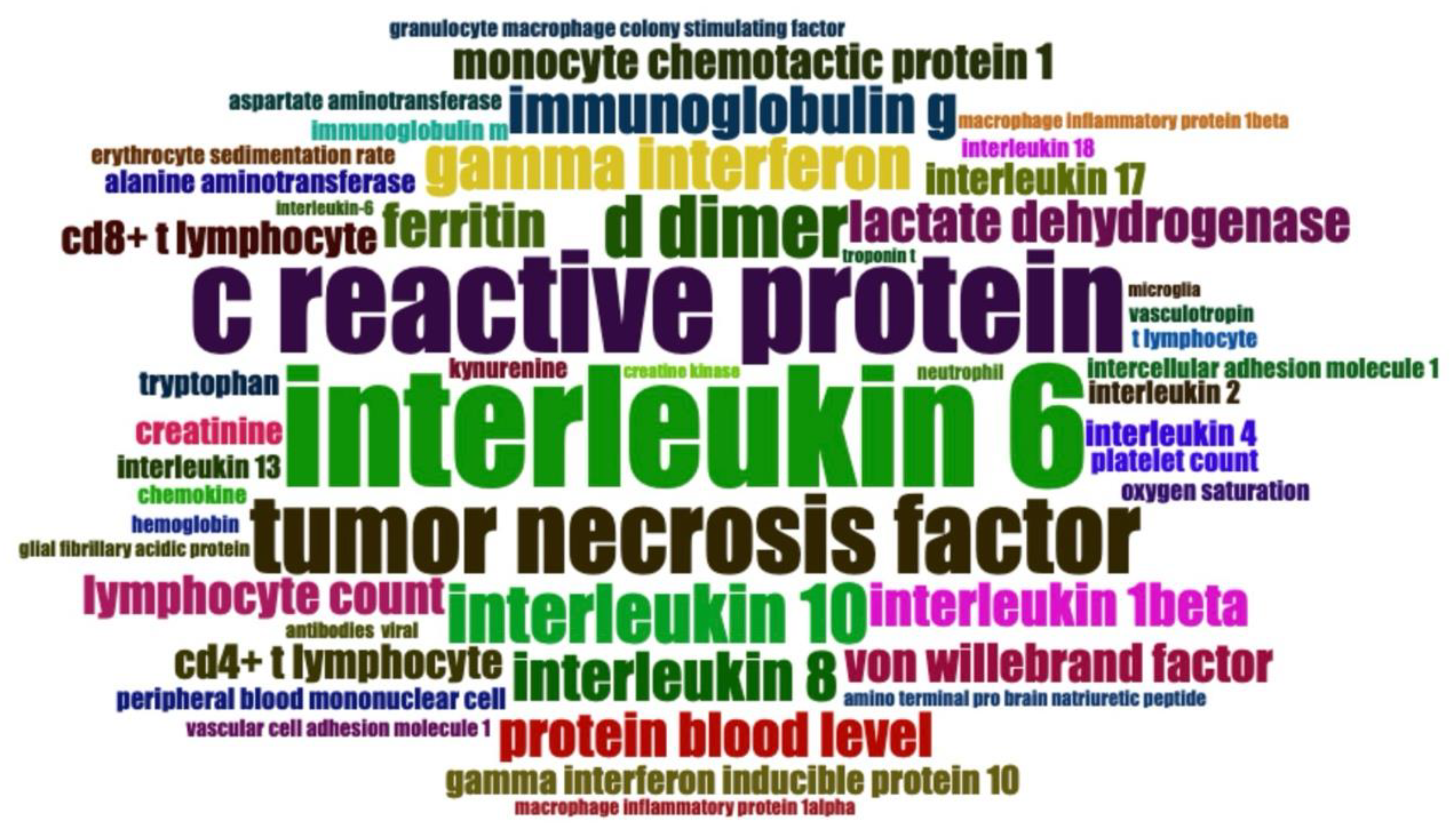
Figure 5.
Word Cloud of Key Biomarkers Associated with Long COVID Research. This word cloud displays the most frequently cited biomarkers in Long COVID research. Larger font size indicates higher mention frequency across studies. Immune and inflammatory markers, such as interleukins and cytokines, appear prominently, suggesting that immune dysregulation is a key area of focus. Different colors help visually distinguish the terms but do not imply specific categories or relevance levels.
Figure 5.
Word Cloud of Key Biomarkers Associated with Long COVID Research. This word cloud displays the most frequently cited biomarkers in Long COVID research. Larger font size indicates higher mention frequency across studies. Immune and inflammatory markers, such as interleukins and cytokines, appear prominently, suggesting that immune dysregulation is a key area of focus. Different colors help visually distinguish the terms but do not imply specific categories or relevance levels.
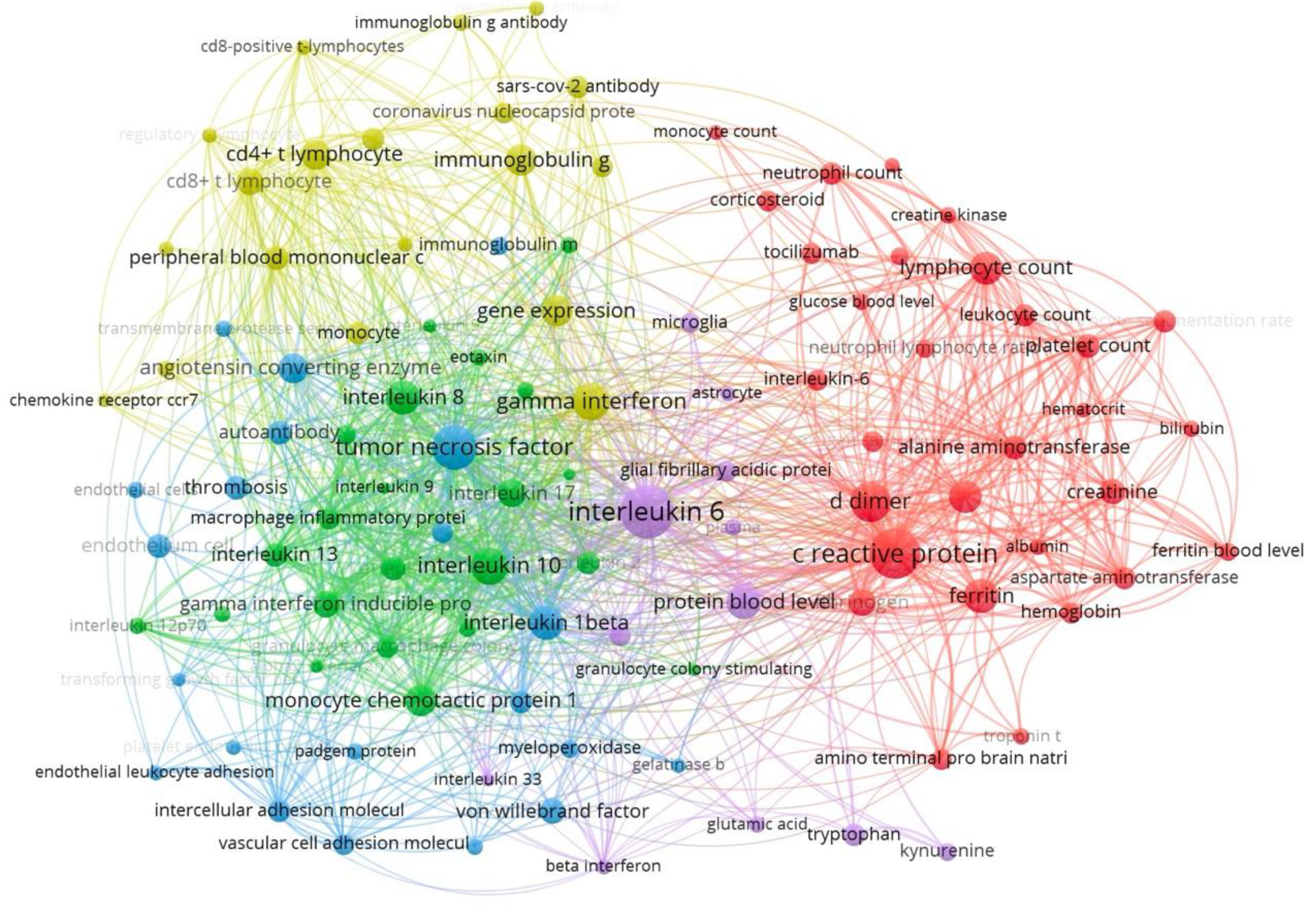
Figure 6.
Keyword co-occurrence network analysis in Long COVID diagnostic biomarker research. This network visualization shows the co-occurrence relationships among frequently cited keywords in Long COVID diagnostic biomarker research. Each node represents a specific keyword, with larger nodes indicating higher usage frequency. Colors group terms into clusters of closely related concepts, while the connecting lines (edges) reflect how often the keywords appear together—thicker lines denote stronger co-occurrence. Notably, immune and inflammatory terms (e.g., interleukin-6, c-reactive protein) form dense clusters, suggesting a strong research focus on immune dysregulation in Long COVID.
Figure 6.
Keyword co-occurrence network analysis in Long COVID diagnostic biomarker research. This network visualization shows the co-occurrence relationships among frequently cited keywords in Long COVID diagnostic biomarker research. Each node represents a specific keyword, with larger nodes indicating higher usage frequency. Colors group terms into clusters of closely related concepts, while the connecting lines (edges) reflect how often the keywords appear together—thicker lines denote stronger co-occurrence. Notably, immune and inflammatory terms (e.g., interleukin-6, c-reactive protein) form dense clusters, suggesting a strong research focus on immune dysregulation in Long COVID.
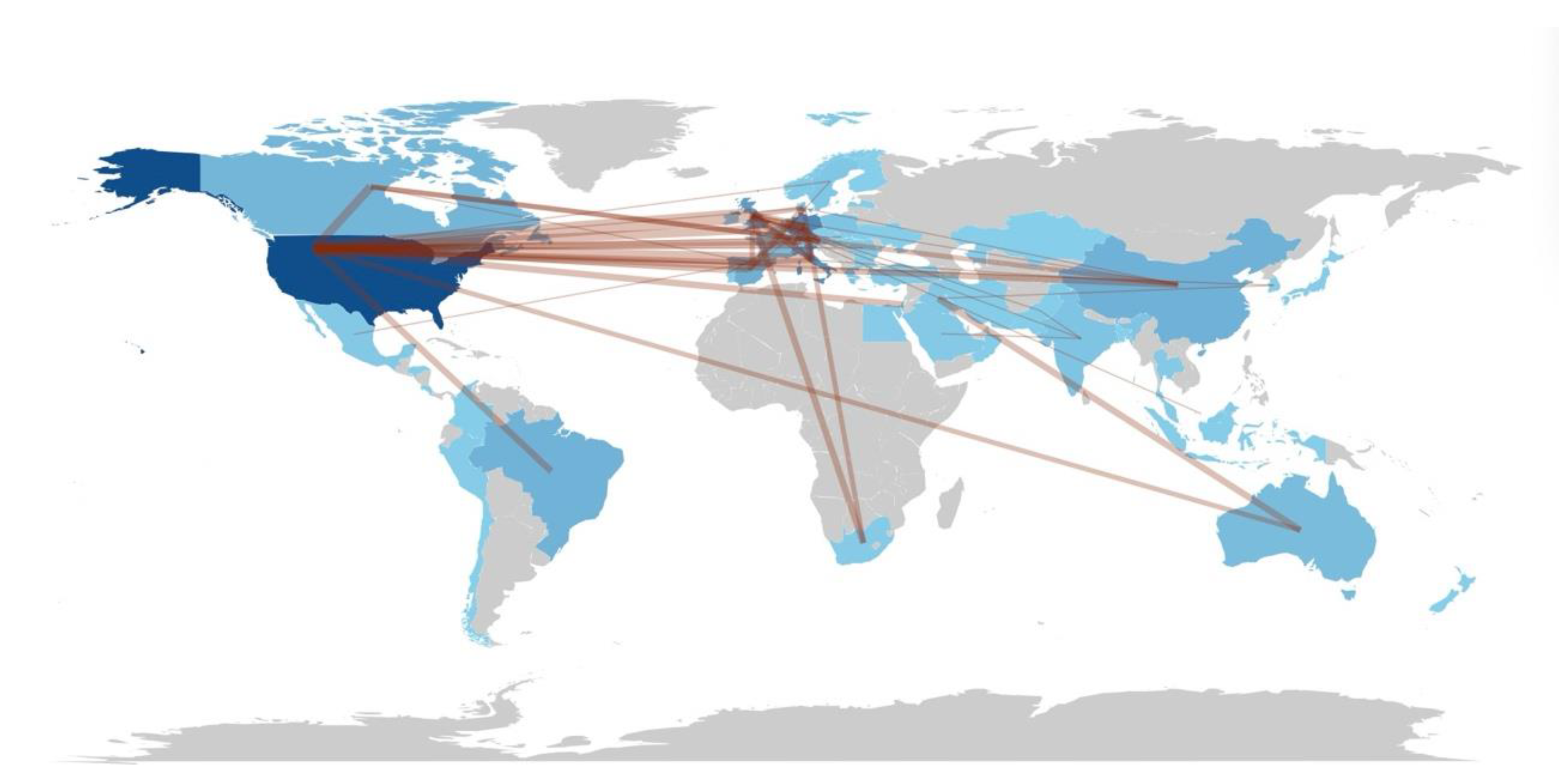
Figure 7.
Global Collaboration Network in Long COVID Diagnostic Biomarker Research. This world map depicts the global collaboration network in Long COVID diagnostic biomarker research. Countries are color-coded by publication volume (darker shading indicates more publications). Lines between countries represent co-authorship links, with thicker lines denoting more frequent or extensive collaborations. Countries without relevant publications appearin a lighter or neutral tone.
Figure 7.
Global Collaboration Network in Long COVID Diagnostic Biomarker Research. This world map depicts the global collaboration network in Long COVID diagnostic biomarker research. Countries are color-coded by publication volume (darker shading indicates more publications). Lines between countries represent co-authorship links, with thicker lines denoting more frequent or extensive collaborations. Countries without relevant publications appearin a lighter or neutral tone.
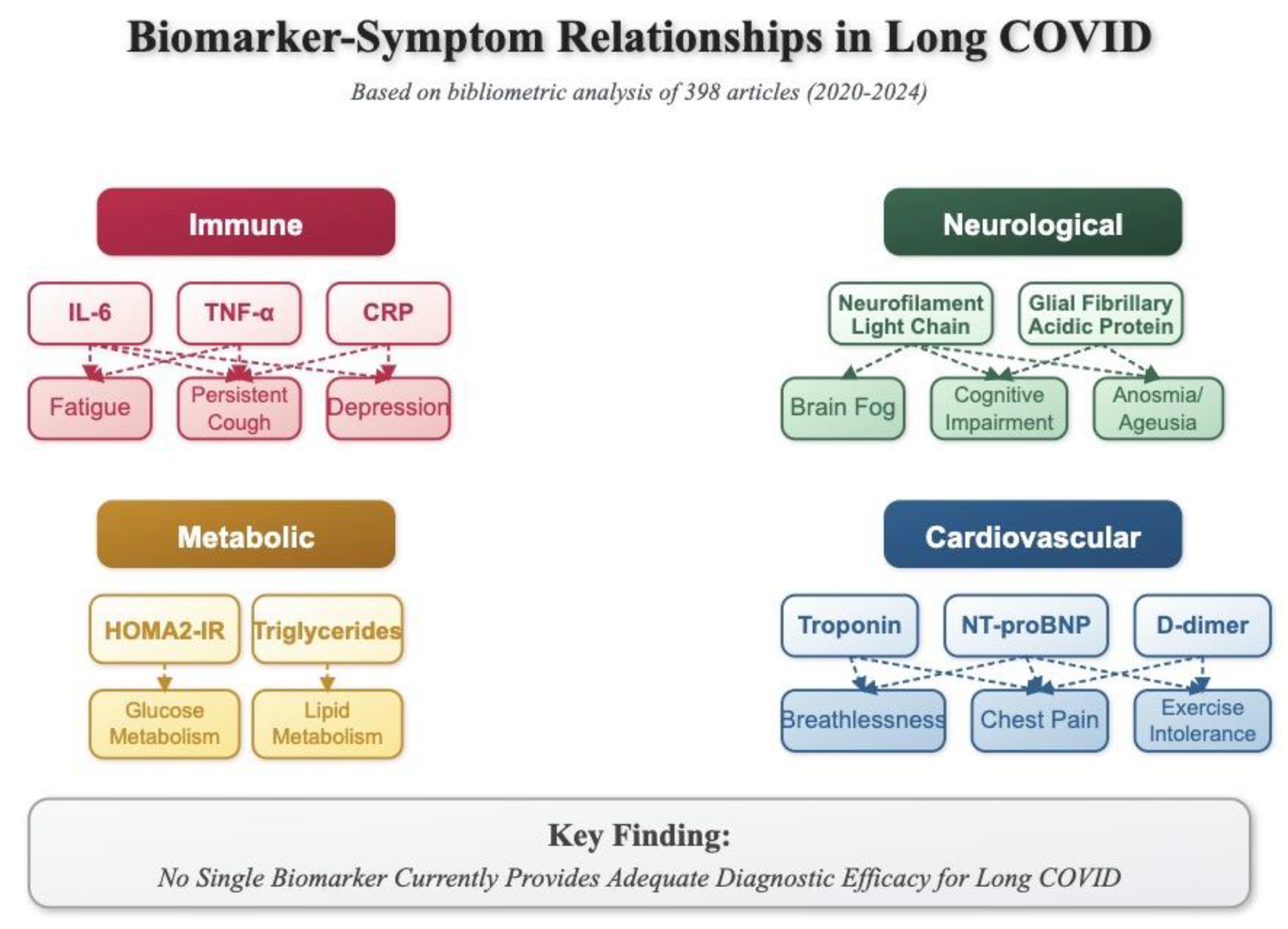
Figure 8.
Biomarker-Symptom Relationships in Long COVID. This schematic illustrates the associations between specific biomarkers and clinical manifestations of Long COVID based on bibliometric analysis of 398 articles (2020-2024). Four major pathophysiological domains are represented: Immune (IL-6, TNF-α, CRP associated with fatigue, persistent cough, and depression), Neurological (neurofilament light chain and glial fibrillary acidic protein linked to brain fog, cognitive impairment, and anosmia/ageusia), Metabolic (HOMA2-IR and triglycerides related to glucose and lipid metabolism dysregulation), and Cardiovascular (troponin, NT-proBNP, and D-dimer correlated with breathlessness, chest pain, and exercise intolerance). The key finding indicates that no single biomarker currently provides adequate diagnostic efficacy for Long COVID, suggesting the need for a multi-biomarker approach in clinical assessment.
Figure 8.
Biomarker-Symptom Relationships in Long COVID. This schematic illustrates the associations between specific biomarkers and clinical manifestations of Long COVID based on bibliometric analysis of 398 articles (2020-2024). Four major pathophysiological domains are represented: Immune (IL-6, TNF-α, CRP associated with fatigue, persistent cough, and depression), Neurological (neurofilament light chain and glial fibrillary acidic protein linked to brain fog, cognitive impairment, and anosmia/ageusia), Metabolic (HOMA2-IR and triglycerides related to glucose and lipid metabolism dysregulation), and Cardiovascular (troponin, NT-proBNP, and D-dimer correlated with breathlessness, chest pain, and exercise intolerance). The key finding indicates that no single biomarker currently provides adequate diagnostic efficacy for Long COVID, suggesting the need for a multi-biomarker approach in clinical assessment.
Table 1.
The 10 most productive countries and institutions in Long -Covid Diagnostic markers research.
Table 1.
The 10 most productive countries and institutions in Long -Covid Diagnostic markers research.
| No. |
Country |
TP |
TC |
The most productive institution |
TPi |
TCi |
| 1 |
United States |
114 |
3390 |
Harvard University |
11 |
417 |
| 2 |
Germany |
60 |
1261 |
Charité – Universitätsmedizin Berlin |
16 |
604 |
| 3 |
Italy |
51 |
685 |
University of Rome La Sapienza |
9 |
144 |
| 4 |
United Kingdom |
43 |
1440 |
University College London |
15 |
832 |
| 5 |
Spain |
26 |
456 |
Centro de Investigación Biomédica en Red |
6 |
174 |
| 6 |
Brazil |
23 |
230 |
Universidade de São Paulo |
11 |
84 |
| 7 |
Canada |
23 |
819 |
University of Alberta |
5 |
57 |
| 8 |
China |
22 |
852 |
University of Zurich |
5 |
131 |
| 9 |
Australia |
18 |
868 |
Barwon Health |
6 |
65 |
| 10 |
France |
15 |
442 |
Institut national de la santé et de la recherche médicale |
12 |
438 |
Table 2.
The 10 most productive authors in Long -Covid Diagnostic markers research topic.
Table 2.
The 10 most productive authors in Long -Covid Diagnostic markers research topic.
| No. |
Author’s name |
TP |
TC |
h-index |
Affiliation |
Country |
| 1 |
Peluso, Michael J. |
7 |
359 |
41 |
University of California at San Francisco |
United States |
| 2 |
Scheibenbogen, Carmen M. |
7 |
303 |
71 |
Charité – Universitätsmedizin Berlin |
Germany |
| 3 |
Quaresma, Juarez A.S. |
7 |
124 |
33 |
Universidade Federal do Pará |
Brazil |
| 4 |
Magno Falcão, Luiz F. |
7 |
124 |
12 |
Universidade do Estado do Pará |
Brazil |
| 5 |
Maes, Michael H.J. |
7 |
91 |
149 |
University of Electronic Science and Technology of China |
China |
| 6 |
Deeks, Steven G. |
6 |
359 |
155 |
University of California at San Francisco |
United States |
| 7 |
Bellmann-Strobl, Judith T. |
6 |
284 |
36 |
Charité – Universitätsmedizin Berlin |
Germany |
| 8 |
Al-Hakeim, Hussein K. |
6 |
65 |
23 |
University of Kufa |
Iraq |
| 9 |
Almulla, Abbas F. |
6 |
65 |
17 |
The Islamic University, Najaf |
Iraq |
| 10 |
Fernández De Las Peñas, César |
6 |
34 |
85 |
Universidad Rey Juan Carlos |
Spain |
Table 3.
The 10 most productive journals in Long -Covid Diagnostic markers research topic.
Table 3.
The 10 most productive journals in Long -Covid Diagnostic markers research topic.
| No. |
Journal |
TP |
TC |
Cite Score 2024 |
The most cited articles |
Publisher |
| 1 |
Frontiers in Immunology |
29 |
358 |
9.8 |
Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning |
Frontiers Media S.A. |
| 2 |
International Journal of Molecular Sciences |
20 |
138 |
6.2 |
Selenium deficiency due to diet, pregnancy, severe illness, or covid-19—a preventable trigger for autoimmune disease |
MDPI |
| 3 |
Journal of Clinical Medicine |
15 |
187 |
4.9 |
Biomarkers of post-COVID depression |
MDPI |
| 4 |
Viruses |
9 |
65 |
5.4 |
Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID |
MDPI |
| 5 |
Scientific Reports |
8 |
31 |
5.6 |
Extended coagulation profile of children with Long COVID: a prospective study |
Nature Publishing Group |
| 6 |
Brain, Behavior, and Immunity |
7 |
811 |
7.7 |
Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis |
Elsevier |
| 7 |
Journal of Medical Virology |
7 |
60 |
4.2 |
Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients |
Wiley-Blackwell |
| 8 |
Cells |
6 |
59 |
6.9 |
Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID |
MDPI |
| 9 |
Frontiers in Medicine |
6 |
130 |
5.1 |
Serum Metabolic Profile in Patients With Long -Covid (PASC) Syndrome: Clinical Implications |
Frontiers Media S.A |
| 10 |
Journal of Personalized Medicine |
6 |
22 |
5.2 |
Long COVID: Clinical Framing, Biomarkers, and Therapeutic Approache |
MDPI |
Table 4.
Top 10 articles on the Scopus database ordered by citation score.
Table 4.
Top 10 articles on the Scopus database ordered by citation score.
| No. |
First Author & Year |
Title |
Journal |
Publisher |
TC 2024 |
| 1 |
Yong, S.J. et al. (2021) |
Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments |
Infectious Diseases |
Sunway University |
653 |
| 2 |
Ceban, F.et al. (2022) |
Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis |
Brain, Behavior, and Immunity |
University Health Network |
581 |
| 3 |
Mandal, S. et al. (2021) |
Long -COVID': A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19 |
Thorax |
Royal Free London Nhs Foundation Trust |
531 |
| 4 |
Phetsouphanh, C. et al. (2022) |
Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection |
Nature Immunology |
University of New South Wales |
444 |
| 5 |
Guedj, E.et al. (2021) |
18F-FDG brain PET hypometabolism in patients with Long COVID |
European Journal of Nuclear Medicine and Molecular Imaging |
Aix-Marseille University |
258 |
| 6 |
Schou, T.M.et al. (2021) |
Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review |
Brain, Behavior, and Immunity |
Aarhus University |
226 |
| 7 |
Spudich, S.et al. (2022) |
Nervous system consequences of COVID-19 |
Science |
Yale School of Medicine |
219 |
| 8 |
Fogarty, H.et al. (2021) |
Persistent endotheliopathy in the pathogenesis of Long COVID syndrome |
Journal of Thrombosis and Haemostasis |
School of Pharmacy and Biomolecular Sciences |
176 |
| 9 |
Gassen, N.C.et al. (2021) |
SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals |
Nature Communications |
University of Bonn |
155 |
| 10 |
Swank, Z. et al. (2023) |
Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae |
Clinical Infectious Diseases |
Harvard Medical School |
146 |
Table 5.
Top 10 cited original articles on biomarkers in Long COVID.
Table 5.
Top 10 cited original articles on biomarkers in Long COVID.
| |
Title |
First Author, Year, Journal |
IF; TC |
Study design, sample number |
Clincial symptom findings, % |
Biomarkers |
| 1 |
Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection |
Phetsouphanh, C.et al, 2022, Nat Immunol. 2022 Feb;23:210-216. |
28.3; 597 |
Cross-sectional study with matched controls, 62 |
Thirty-one patients (21.08%) were defined as having Long COVID (LC), characterized by the persistence of at least one of the following symptoms at 4 months after infection: fatigue, dyspnea, or chest pain. |
BT: IFN-β ↑ (remained elevated in LC); IFN-λ1 ↑ (remained elevated in LC); IL-8 ↓ (decreased in both groups); CXCL9 ↓ (decreased in both groups); CXCL10 ↓ (decreased in both groups); PTX3, IFN-γ, IFN-λ2/3 and IL-6 highly associated with LC |
| 2 |
Long -COVID': A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19 |
Mandal, S., 2021, Thorax. 2021 Apr;76:396-398. |
9; 587 |
Cross-sectional study, 384 |
Persistent breathlessness: 53%
Persistent cough: 34%
Persistent fatigue: 69%
Depression (Patient Health Questionnaire-2 ): 14.6% |
BT: D-dimer ↑ (785 → 384 ng/mL); C-reactive protein ↑ (76 → 1 mg/L); Lymphocytes ↑ (0.95 → 1.94 x109/L); Ferritin ↓ (861 → 169 mcg/L); ALT ↓ (36 → 26 iu/L); AST ↓ (45 → 24 iu/L) |
| 3 |
18F-FDG brain PET hypometabolism in patients with long COVID |
Guedj, E., 2021, Eur J Nucl Med Mol Imaging. 2021 Aug;48:2823-2833. |
8.6; 308 |
Cross-sectional study with matched controls, 79 |
Dyspnea: 80%
Pain: 66%
Memory/cognitive impairment: 49%
Insomnia: 46%
Hyposmia/anosmia: 29%
Dysgeusia/ageusia: 26% |
PET imaging revealed significant hypometabolism in the following brain regions compared to healthy controls:
Bilateral rectus/orbital gyrus ↓ (including olfactory gyrus); Right temporal lobe ↓ (including amygdala and hippocampus); Bilateral pons/medulla brainstem ↓; Bilateral cerebellum ↓ |
| 4 |
Distinguishing features of long COVID identified through immune profiling |
Klein et al., 2023, Nature. 2023 Nov;623:139-148. |
54.4; 291 |
Cross-sectional study, 275 |
Fatigue: 87%
Brain fog: 78%
Memory difficulty: 62%
Confusion: 55% |
BT: Non-conventional monocytes ↑; cDC1 cells ↓;
CD4+ IL-4/IL-6 double-positive T cells ↑; Anti-S1 IgG levels ↑; Anti-N IgG levels ↑; EBV gp23 antibodies ↑; Cortisol ↓; Complement C4b ↑; CCL19 ↑; Galectin-1 ↑ |
| 5 |
Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae |
Swank, Z., 2023, Clin Infect Dis. 2023 Feb 8;76:e487-e490. |
8.2; 246 |
Retrospective pilot study with controls, 63 |
Cardiovascular symptoms
Systemic symptoms
Head-eye-ear-nose-throat symptoms
Musculoskeletal symptoms |
BT: Spike ↑ (60% PASC vs 0% COVID-19); S1 subunit ↑ (~20% PASC vs rare in COVID-19); Nucleocapsid ↑ (1 PASC patient vs rare in COVID-19) |
| 6 |
Persistent endotheliopathy in the pathogenesis of long COVID syndrome |
Fogarty, H., 2021, J Thromb Haemost. 2021 Oct;19:2546-2553. |
5.5; 211 |
Cross-sectional study with controls, 67 |
Required hospitalization: 74%
Required ICU admission: 16%
Comorbidities present: 62% |
BT: Factor VIII:C levels ↑
von Willebrand factor antigen ↑ ; von Willebrand factor propeptide ↑
Soluble thrombomodulin ↑; Thrombin Generation:
Lag times ↓; Endogenous thrombin potential ↑; Peak thrombin ↑ |
| 7 |
Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection |
Peluso, M. J., 2021, J Infect Dis. 2021 Dec 1;224:1839-1848. |
5; 173 |
Prospective cohort study, 121 |
Concentration problems: 57.5%
Fatigue: 56.2%
Sleep problems: 43.8%
Anosmia/dysgeusia: 37.0% |
BT: TNF-α ↑; IP-10 ↑; IL-6 ↑ |
| 8 |
A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity |
Kedor et al., 2022, Nat Commun. 2022 Aug 30;13:5104. |
16.1; 164 |
Prospective observational cohort study, 42 |
Fatigue: 100%
Post-exertional malaise: 100%
Need for rest: 96-100%
Cognitive impairment: 91%
Mental fatigue: 100%
Sleep disturbances: 83-89% |
BT: Interleukin-8 in erythrocytes ↑; Angiotensin converting enzyme 1 ↓; Mannose binding lectin ↓; Antinuclear antibodies (elevated 1:160-1:1280) ↑ |
| 9 |
Long -term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms |
Peluso, M. J., 2021, Cell Rep. 2021 Aug 10;36:109518. |
7.5; 149 |
Prospective observational cohort study, 70 |
Persistent symptoms at first visit: 45.8%
Persistent symptoms at 4 months: 53.8%
Neurological symptoms: >70%
Fatigue & reduced exercise tolerance: >70%
Loss/change in smell/taste: >70%
Pulmonary symptoms: >70% |
BT: CD4+ T cells ↑; N-specific interferon-γ producing CD8+ T cells ↓; CD8+ T cells expressing CD107a ↓; IP-10 ↑; Neutralizing antibodies ↓ |
| 10 |
Prolong ed elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response |
Townsend, L., 2021, J Thromb Haemost. 2021 Apr;19:1064-1070. |
5.5; 135 |
Cross-sectional observational study, 150 |
Fatigue: 51%
Breathlessness and reduced exercise tolerance
Abnormal chest x-rays (significantly more common in elevated D-dimer group) |
BT: D-dimer ↑ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).