1. Introduction
Psoriasis is a chronic, immune-mediated inflammatory disease primarily manifesting as skin lesions. It is driven by the interaction of genetic and environmental factors, leading to an aberrant immune response. Beyond dermatological involvement, psoriasis is increasingly recognized as a systemic disorder capable of affecting multiple organs, including joints, cardiovascular structures, and musculoskeletal components such as tendons and entheses [
1]. Enthesitis, defined as inflammation at the insertion sites of tendons, ligaments, or joint capsules into bone, frequently affects the Achilles tendon in patients with psoriasis. Such involvement may represent an early indicator of psoriatic arthritis (PsA). According to Fiorenza et al. (2021), asymptomatic enthesitis at the Achilles tendon insertion site is detected via ultrasound in approximately 30–50% of psoriasis patients, which might later progress to clinical symptoms [
2].
When psoriasis patients experience Achilles tendon-related pain, potential causes include tendinitis and neuropathic pain from peripheral nerve entrapment. Fascial tissues are critical in nerve entrapment syndromes, often contributing to nerve compression and pain. The crural fascia, a deep fascial layer of the lower leg, surrounds the calf muscles and forms compartments and peripheral nerves travels with the fascia, e.g. the sural nerve in the fascia between the medial and lateral gastronemius. Its primary function is to provide structural support and facilitate muscle action. Diagnosing these conditions can be challenging due to overlapping symptoms with other musculoskeletal disorders and the limitations of traditional imaging techniques. Traditional methods like X-rays primarily visualize bony structures and are unable to depict soft tissue abnormalities such as fascial thickening, adhesions, or subtle nerve compressions [
3]. MRI can visualize soft tissues, but its resolution may be insufficient to detect subtle fascial restrictions or early-stage nerve entrapment, and it often lacks the dynamic assessment capabilities needed to evaluate nerve mobility during movement [
4].
The DANBIO study, a Danish registry for biological treatment in rheumatology—a clinical quality assurance database initiated in 2000—reported a notably higher prevalence of neuropathic pain among PsA patients compared to those with rheumatoid arthritis (RA) or other spondyloarthritis (SpA) [
5]. This study utilized the PainDETECT questionnaire (PDQ) to evaluate neuropathic pain components, finding a notably higher prevalence of neuropathic pain features in PsA patients (28%) compared to those with RA (17%) and axial SpA (13%), suggesting neuropathic pain as a clinically significant issue in PsA [
6,
7]. However, the clinical or imaging parameters of neuropathic pain in PsA patients remain underexplored. Given this, it is important to recognize that chronic foot and ankle pain—frequently reported among patients with musculoskeletal or systemic inflammatory conditions—may arise from diverse etiologies, including pathologies of ligaments, tendons, nerves, vascular structures, or skin. Among these, nerve entrapment syndromes such as sural nerve entrapment, although relatively uncommon, should not be overlooked as potential contributors to persistent heel or lateral foot pain [
8,
9,
10,
11,
12]. Traditional diagnostic methods, such as physical examination and standard radiography, often lack the sensitivity to detect subtle fascial abnormalities contributing to nerve entrapment. Ultrasound imaging offers a valuable alternative for visualizing these structures and guiding interventions.
US-guided HD has emerged as an effective treatment for persistent foot pain, particularly in cases resistant to conservative treatment. This technique involves the injection of fluid around nerves to physically separate them from adhesions, thereby relieving pressure on nervi nervorum and vasa nervorum, ultimately alleviating pain and improving function [
13]. Recent case reports indicate that sural nerve entrapment, unresponsive to conservative treatment, can be safely and effectively managed through US-guided HD [
8,
14,
15,
16], a finding consistent with positive outcomes observed in other peripheral nerve conditions such as carpal tunnel syndrome [
8,
16,
17]. Additionally, high-volume image-guided injection (HVIGI), which targets increased neovascularization and nerve infiltration in chronic Achilles tendinopathy, has demonstrated significant clinical efficacy [
18,
19,
20].
Based on these findings, we report a case of sural nerve entrapment mimicking Achilles tendinopathy in a patient with psoriasis, successfully managed by US-guided HD performed below the psoriatic lesion. The patient experienced substantial pain relief and functional improvement, highlighting the therapeutic potential of US-guided HD for neuropathic Achilles pain associated with psoriasis.
2. Case Presentation
A 41-year-old Korean male, employed as a warehouse worker, presented with stiffness and paresthesia in his right heel area. These symptoms had gradually worsened over two months and rapidly aggravated over the previous two days. The patient reported severe pain rated as 8 on a numeric pain rating scale (NPRS), accompanied by morning stiffness and muscle tightness, significantly impairing his daily activities. He exhibited an antalgic gait due to pain. He had no previous history of similar symptoms or recent trauma.
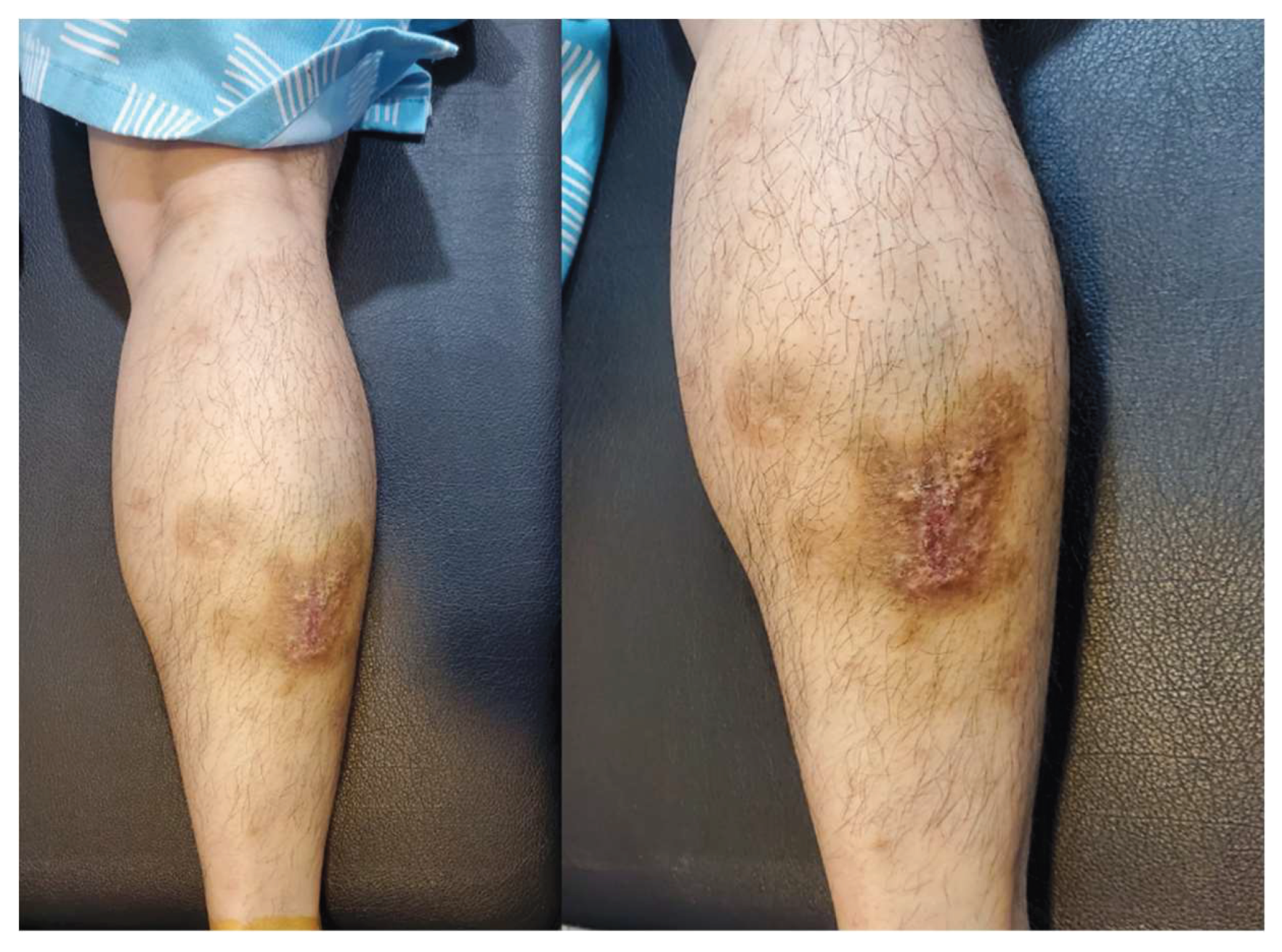
On physical examination, the patient demonstrated tenderness in the posterior calcaneal area and discomfort upon dorsiflexion of the right ankle. A psoriatic skin lesion was observed on the right calf (
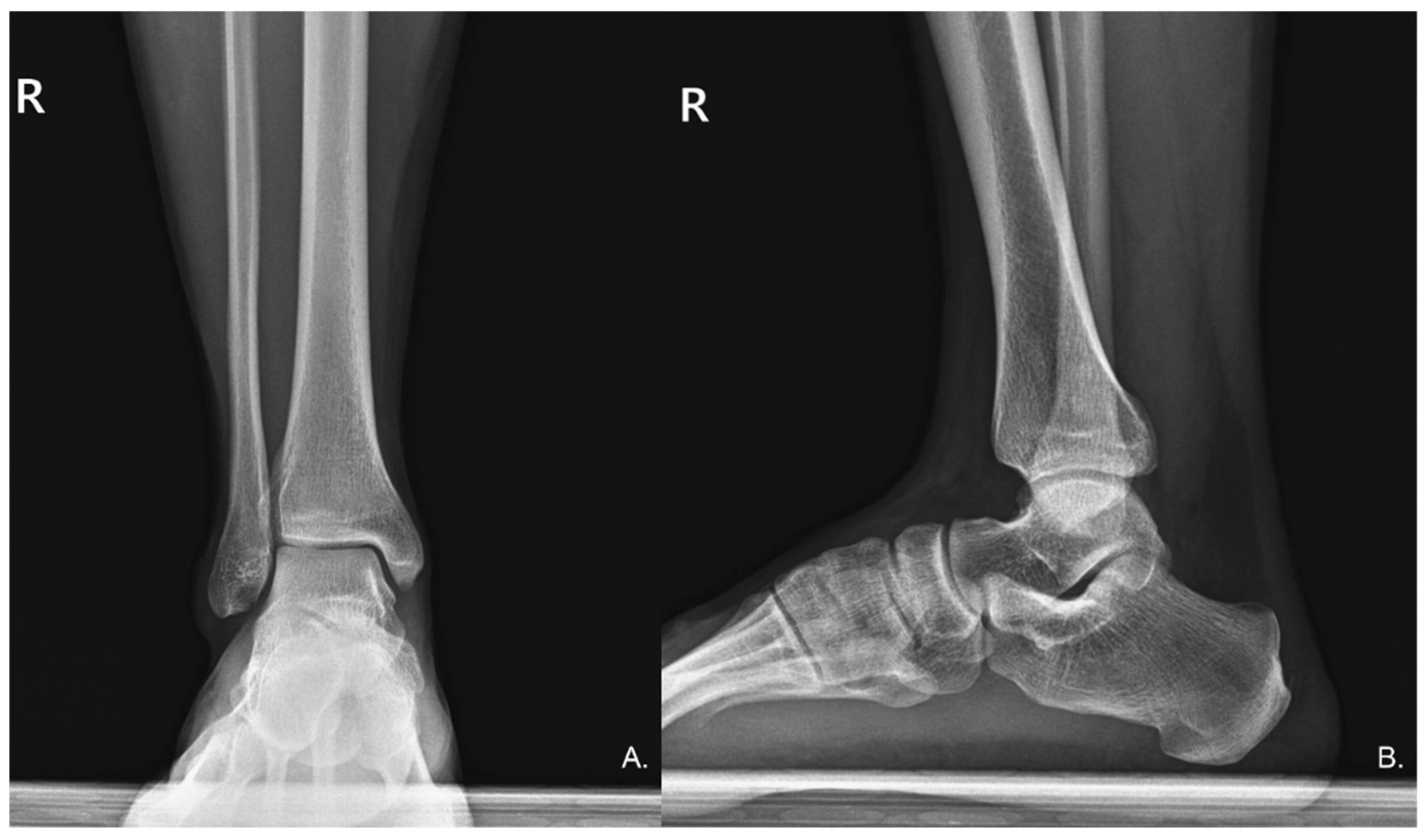
Figure 1), and the patient disclosed a prior diagnosis of psoriasis six years earlier, with no ongoing treatment. To rule out bony deformities that could cause Achilles tendon pain, anteroposterior and lateral ankle radiographs were obtained, which revealed no radiographic abnormalities (
Figure 2). Additionally, impaired balance during a single-leg stance on the right foot was noted.
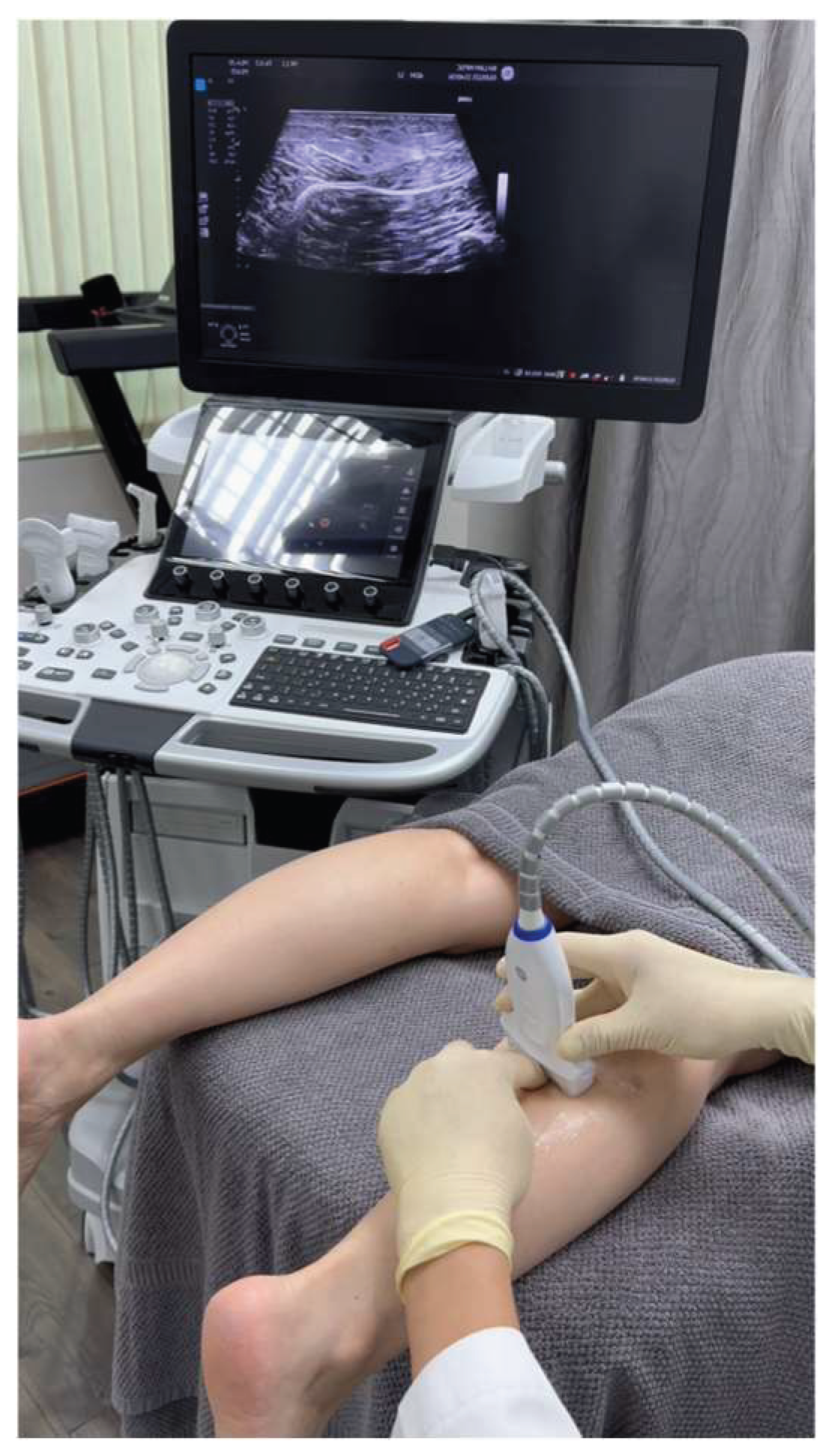
While ultrasound evaluation of the Achilles tendon demonstrated no intrinsic structural abnormalities, Sonoguide Digital Palpation (SDP) of the sural nerve, performed under ultrasound guidance, provided key diagnostic information. SDP reproduced the patient’s characteristic Achilles tendon pain, suggesting sural nerve entrapment. Furthermore, SDP revealed decreased mobility of the sural nerve within the fatty triangle between the medial and lateral gastrocnemius muscles, specifically within the crural fascia external to the gastrocnemius. Additional findings included increased fibrous tissue within this fatty triangle and increased signal indicative of edema distal to the suspected entrapment site, all of which strongly supported the diagnosis of sural nerve entrapment (
Figure 3, video 1). Prior to this diagnosis, the patient had failed to respond to conservative treatments, including three courses of physiotherapy and pharmacological therapy with NSAIDs and pregabalin.
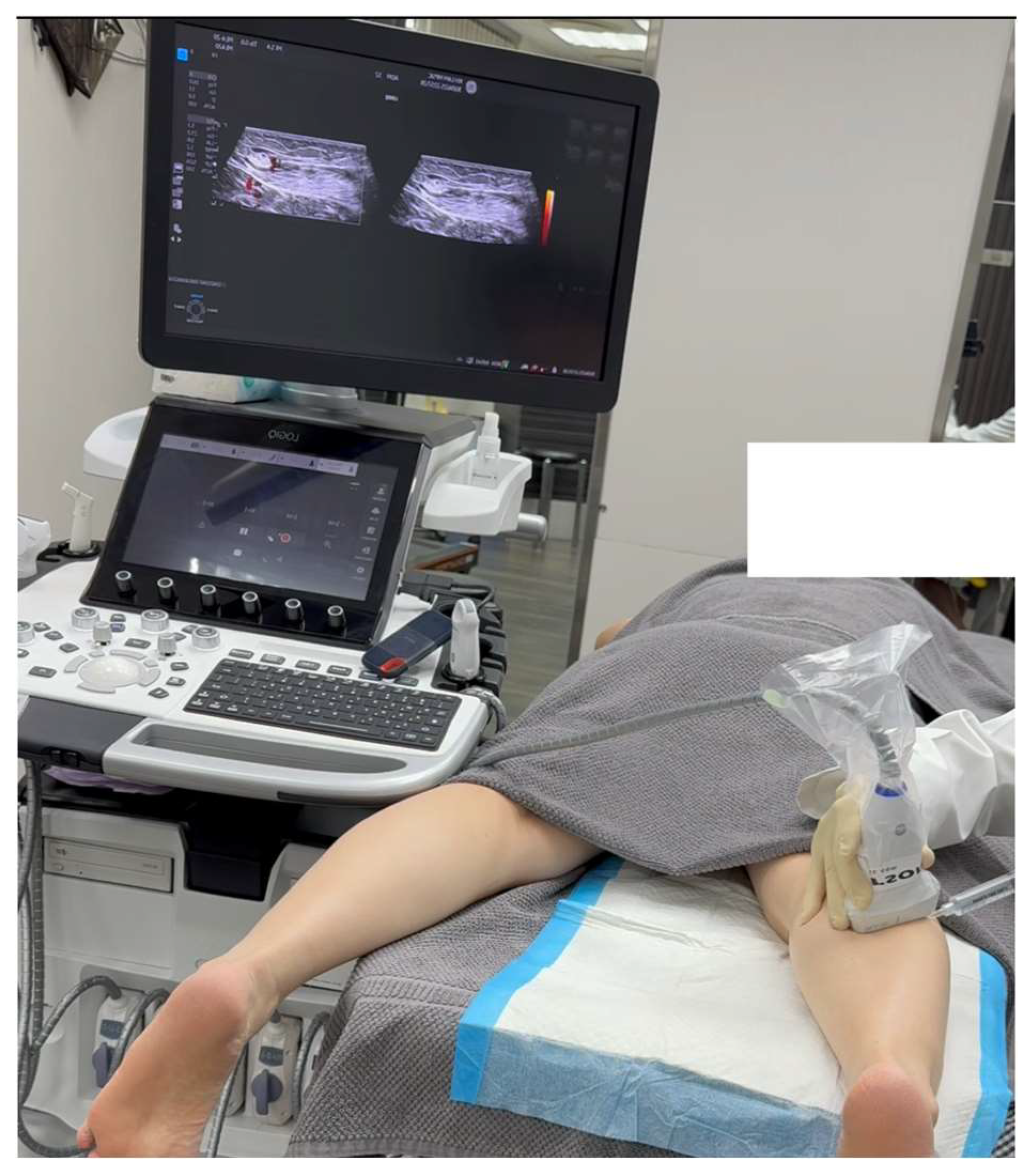
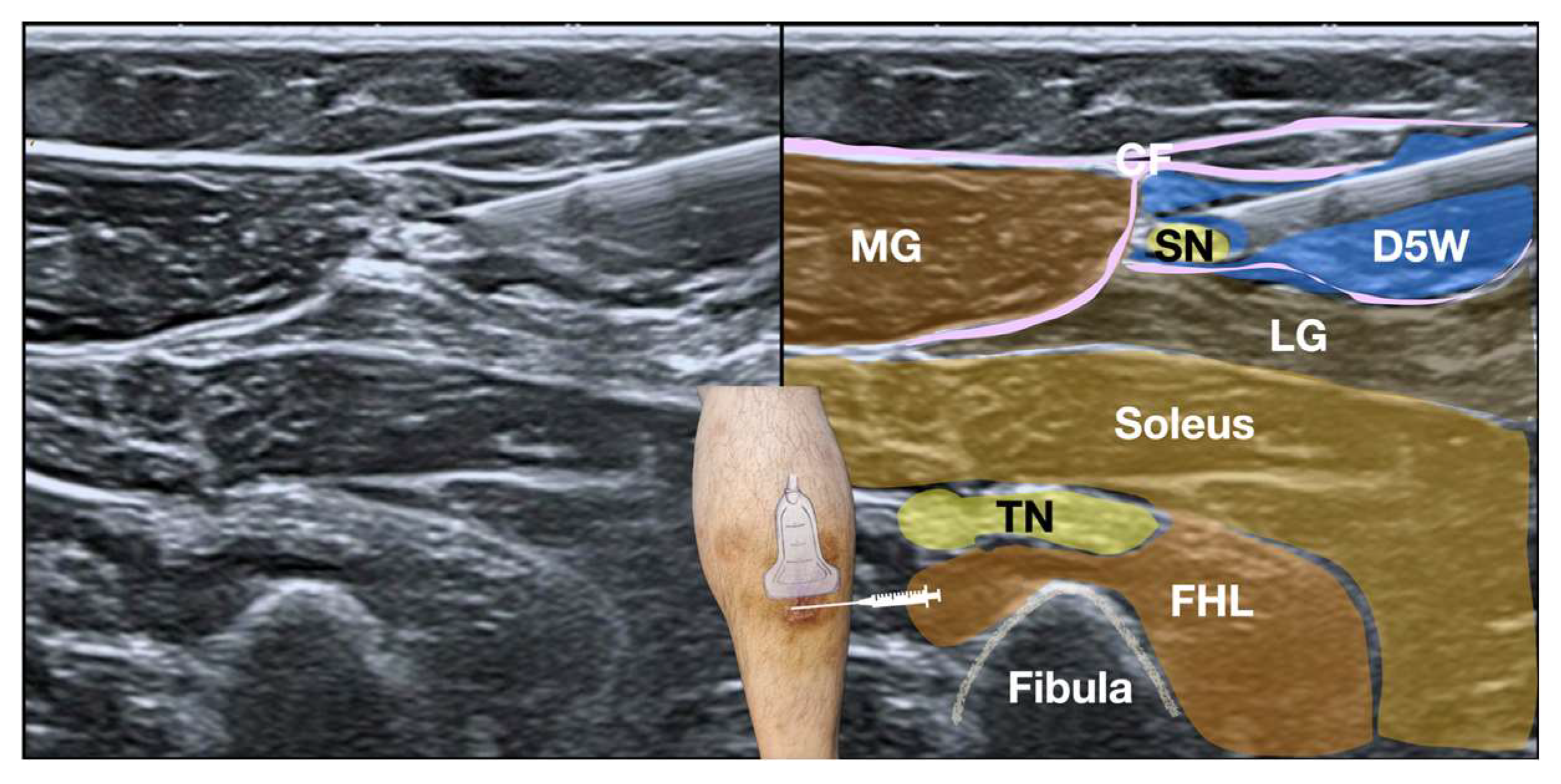
Given the failure of conservative management and the SDP-confirmed diagnosis of sural nerve entrapment, ultrasound-guided hydrodissection (HD) was performed using 50 cc of 5% dextrose in water (D5W) without local anesthetics. The D5W was administered via a lateral-to-medial approach below the psoriatic skin lesion on the right calf to alleviate the symptoms (
Figure 4 and
Figure 5, Video 2). Immediately post-procedure, the patient reported significant pain relief, with a notable reduction in NPRS scores from 8 to 2 during ambulation. Follow-up conducted via a telephone interview at 18 months post-intervention confirmed continued symptom relief and complete functional recovery.
This retrospective case report qualified for exemption from Institutional Review Board (IRB) review because it involved retrospective analysis of anonymized clinical data and documents, with no direct patient contact or collection of personally identifiable information after the initial clinical encounter. Informed consent for the publication of clinical details and outcomes was obtained from the patient via telephone interviews. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
2.1. Diagnosis of Sural Nerve Entrapment Using Sonoguide Digital Palpation
Sonoguide Digital Palpation was used to assist in diagnosing and visualizing the suspected lesions associated the sural nerve entrapment (
Figure 3 and Video 1).
Video 1. Real-time ultrasound imaging during Sonoguide Digital Palpation (SDP) of the sural nerve (SN) in this patient with psoriasis and Achilles pain, demonstrating features of SN en-trapment. The red arrow indicates the fatty triangle within the crural fascia, where the SN and lesser saphenous vein (LSV) are located. Note the increased hyperechoic fibrotic tissue and de-creased mobility of the SN within the fatty triangle, indicative of entrapment. Anechoic signals surrounding the fatty triangle suggest local edema. The video correlates the palpating finger’s movements with the real-time ultrasound findings, allowing for precise assessment of the SN’s condition and the patient’s pain response during SDP. The video demonstrates the restricted movement of the SN within the fibrotic tissue, and the reproduction of the patient’s Achilles tendon pain upon palpation.
https://www.dropbox.com/scl/fi/ho23g5pm7fkev5nhiawgc/Sn-SDP-arrowed.mp4?rlkey=uqhx4n5jifkqa2gmrzoaha6yu&dl=0
Sonoguide Digital Palpation (SDP), is a dynamic technique that utilizes layered palpation under ultrasound guidance [
21,
22]. This allows for the visualization and diagnosis of changes in tissue tension and friction. In this case, SDP was used to assess the crural fascia (CF). Excessive displacement or gliding restriction of the CF relative to the superficial adipose tissue and the underlying gastrocnemius muscle may signify fascial defects (Video 1). A key advantage of this technique is the ability to perform real-time comparison with the contralateral side as a control.
The amount of pressure applied during SDP was firm but controlled, typically between 2-4 kg/cm², as estimated by tactile feedback and clinical experience. Rhythmic palpation was performed for approximately 10-15 seconds at each location, with alternating lateral-to-medial and medial-to-lateral movements. The criteria used to determine nerve entrapment included:
* Reproduction of the patient’s characteristic Achilles tendon pain with palpation over the sural nerve.
* Palpable tethering or decreased mobility of the sural nerve compared to the surrounding tissues.
* Increased resistance to gliding of the sural nerve during palpation.
* Visualization of increased fibrotic tissue or edema surrounding the sural nerve on ultrasound.
SDP was also selectively applied to palpate the sural nerve (SN) and the lesser saphenous vein (LSV) within the fatty triangle of the crural fascia, located superficially between the medial and lateral gastrocnemius muscles. During SDP, digital compression over the fatty triangle allows for assessment of the relative mobility of the SN and LSV in relation to surrounding structures. The presence of excessive fibrotic tissue, tethering of the SN to the surrounding tissues, and reproduction of the patient’s concordant pain can help confirm SN entrapment. Furthermore, layered digital palpation was used to assess for myofascial trigger points in the medial and lateral gastrocnemius and soleus muscles, using varying depths and forces. Visualization of a twitch response in these muscles upon deeper and sustained pressure may further assist in differentiating the root cause of the calf pain.
2.2. Ultrasound-Guided Hydrodissection for Sural Nerve Entrapment
Given the patient’s failure to respond to previous conservative treatments and the suspected sural nerve (SN) entrapment identified through Sonoguide Digital Palpation (SDP), ultrasound-guided hydrodissection of the SN was proposed. Informed consent was obtained, and the patient agreed to participate in this study.
Procedure: A linear high-frequency transducer (10-20 MHz) was used for the procedure. The skin was prepared in a sterile manner with 2% chlorhexidine in 75% alcohol [
23], and the transducer was covered with a sterile probe cover. To enhance patient comfort, local anesthesia was administered to the skin prior to hydrodissection. A 25-gauge x 2-inch hypodermic needle was used, along with 50 cc of 5% dextrose in water (D5W) without local anesthetic as the hydrodissection solution (
Figure 4 and
Figure 5, and Video 2).
The hydrodissection technique relies on the injectate to "dissect" and separate the soft tissues ahead of the needle tip, creating a halo. The needle is then advanced, following this halo to a new position. Hydrodissection was initiated at the bottom of the SN with the needle bevel facing upward, followed by hydrodissection of the top of the SN with the needle bevel facing downward. The goal was to achieve complete circumferential hydrodissection of the SN, with the injectate surrounding the nerve above, below, medially, and laterally.
The SN was initially hydrodissected at the site of maximal tenderness and restricted nerve mobility identified during SDP. Specifically, this location corresponded to the area where palpation most consistently reproduced the patient’s concordant Achilles tendon pain, and where ultrasound imaging revealed the greatest degree of fibrotic tissue and tethering of the nerve to the surrounding crural fascia. This process was then repeated, using the same needle entry point, with the transducer and needle pivoted proximally and then distally along the course of the SN. The aim was to hydrodissect the SN over a sufficient length to achieve complete release of the entrapment.
Video 2. Ultrasound-guided hydrodissection of the sural nerve. The video demonstrates the in-plane approach for hydrodissection, with the needle (arrow) visualized as it advances towards the sural nerve (SN). The injectate (5% dextrose in water, D5W) is seen expanding around the SN, achieving circumferential re-lease. Anatomical landmarks include: MG - medial gastrocnemius, LG - lateral gastrocnemius, FHL - flexor hallucis longus, TN - tibial nerve. Note the improved mobility of the SN following hydrodissection. The video clearly shows the injectate separating the SN from the surrounding fibrotic tissue, resulting in improved nerve gliding.
Sonoguide Digital Palpation (SDP) reproduced the patient’s concordant pain, confirming sural nerve (SN) involvement. Following hydrodissection with 50 cc of D5W, the SN was completely released within the fatty triangle with the injectate surrounding the medial, lateral, anterior and posterior to the SN. Real-time ultrasound imaging confirmed circumferential distribution of the injectate around the sural nerve, indicating complete release from surrounding tissues. Immediately post-procedure, the patient reported significant pain relief, with a notable reduction in NPRS scores from 8 to 2 during ambulation. The patient also reported resolution of the stiff sensation previously experienced over the Achilles tendon. Post-hydrodissection SDP over the previous SN entrapment region no longer reproduced the patient’s Achilles tendon pain and revealed improved mobility of the SN within the fatty triangle. The improvement in SN mobility was assessed subjectively based on the ease with which the nerve could be displaced with gentle pressure during SDP, as visualized on real-time ultrasound. Prior to hydrodissection, the nerve exhibited significant tethering and resistance to displacement, whereas post-hydrodissection, the nerve demonstrated a noticeably smoother and more fluid gliding motion relative to the surrounding tissues. Notably, an 18-month follow-up was conducted via telephone interview, during which the patient confirmed sustained symptom relief and complete functional recovery.
3. Literature review: Psoriasis and Sural Nerve Entrapment
3.1. Ultrasound-Guided Hydrodissection for Sural Nerve Entrapment
Sural nerve entrapment is a cause of lateral ankle and foot pain that can be challenging to diagnose and treat. Conservative treatments often fail, leading to the exploration of interventional techniques. Ultrasound-guided hydrodissection has emerged as a promising minimally invasive approach for managing sural nerve entrapment.
To date, only case reports and small studies suggest the potential efficacy of ultrasound-guided hydrodissection for sural neuropathy. Fader et al. (2015) presented a case where ultrasound imaging revealed sural nerve entrapment with edema and scar formation, which was successfully treated with percutaneous ultrasound-guided hydrodissection [
16]. Similarly, Tople (2021) reported a case of sural nerve entrapment effectively treated with ultrasound-guided hydrodissection [
15]. Omodani (2023) also supports the use of hydrodissection where localized tenderness around the sural nerve was observed [
14]. Bhuyan and Tople (2021) performed percutaneous ultrasound-guided hydrodissection of the sural nerve, with the patient reporting complete improvement in pain [
8,
15].
These reports highlight the use of ultrasound to visualize the sural nerve, identify potential entrapment sites (often associated with edema or scar tissue), and guide the precise injection of fluid to release the nerve from surrounding tissues. The mechanism of action is thought to involve mechanical separation of the nerve from adhesions, 2potentially relieving compression of the nervi nervorum and vasa nervorum.
The current literature is limited to case reports and small case series. Larger, controlled studies are needed to determine the true efficacy of ultrasound-guided hydrodissection for sural nerve entrapment, to compare it to other treatment options, and to identify factors that predict successful outcomes. Further research is also needed to optimize the hydrodissection technique (e.g., the volume and type of injectate used) and to better understand the long-term effects of the procedure.
3.2. Psoriasis and Nerve Entrapments:
To date, no studies have specifically investigated the use of US-guided HD for SN entrapment in patients with psoriasis. However, psoriasis, recognized as an immune-mediated inflammatory disease with systemic manifestations beyond the skin [
1], may contribute to nerve entrapment through several mechanisms.
Inflammation of Surrounding Tissues: Psoriasis is characterized by the excessive production of pro-inflammatory cytokines, including TNF-alpha [
24]. This systemic inflammation can extend beyond the skin, affecting surrounding tissues such as muscles, tendons, and fascia. Inflammation within these tissues can result in swelling and thickening, potentially compressing adjacent nerves and leading to entrapment. For instance, psoriatic inflammation and subsequent thickening of the crural fascia surrounding the sural nerve could directly compress the nerve, causing pain, paresthesia, and other symptoms associated with SN entrapment.
Fascial Changes: Chronic inflammation associated with psoriasis may induce alterations in fascial structure and function. Fascia, a connective tissue enveloping muscles, nerves, and other structures, can become fibrotic (scarred) and less flexible due to chronic inflammation. This reduced fascial mobility can restrict nerve gliding and increase the susceptibility to entrapment [
25,
26,
27].
Enthesitis and Joint Involvement: Psoriatic arthritis (PsA), a common comorbidity affecting approximately one-third of psoriasis patients [
1,
26,
28], is characterized by inflammation at the entheses (tendon and ligament insertion sites into bone) and within the joints [
24,
27]. This inflammation can lead to periarticular swelling and structural changes, potentially compressing nearby peripheral nerves.
Neurogenic Inflammation: Emerging evidence suggests a role for the nervous system in psoriasis, with increased innervation of sensory nerve fibers and the presence of neurogenic inflammation [
29]. This may establish a feedback loop where nerve inflammation contributes to skin inflammation, and vice versa, potentially exacerbating nerve entrapment.
Proximity of Inflamed Structures to Nerves: Inflammation in one anatomical structure can readily spread to adjacent structures [
27,
28]. Consequently, if psoriatic inflammation occurs in tissues in close proximity to a nerve, the inflammation can directly affect the nerve, causing irritation or compression.
In summary, psoriasis-related inflammation can affect various tissues surrounding peripheral nerves, potentially leading to compression, restricted movement, and ultimately, nerve entrapment. The chronic nature of psoriasis and its systemic inflammatory effects suggest that it may be a contributing factor to nerve entrapment syndromes.
4. Discussion
In the present case, we demonstrated significant improvement in Achilles tendinopathy-related pain in a patient with psoriasis through US-guided HD of the lateral sural nerve beneath a psoriatic skin lesion. Unlike previous approaches that targeted direct Achilles tendon intervention, our method focused explicitly on releasing proximal nerve entrapment. Although prior studies have shown favorable outcomes with HVIGI directly into the Achilles tendon, primarily addressing tendon neovascularization and nerve ingrowth [
18,
30], our intervention highlights a distinct therapeutic mechanism involving both mechanical and biochemical effects of HD around the proximal sural nerve.
The efficacy of US-guided HD in nerve entrapment syndromes is increasingly recognized, despite being relatively understudied. This intervention involves mechanically separating entrapped nerves from surrounding fascia, potentially relieving mechanical compression and associated neuropathic symptoms [
31]. The surrounding fascia, when thickened or adhered, can directly contribute to nerve compression, highlighting the importance of addressing fascial pathology in these cases. Bhuyan et al. (2021) demonstrated substantial and sustained pain relief in patients with refractory sural nerve entrapment following US-guided HD, noting no recurrence. Lam et al. (2020) proposed a mechanism involving mechanical separation of perineural adhesions, alleviating compression of the nervi nervorum and vasa nervorum — structures crucial for neural innervation and vascular supply. In addition to these mechanical effects, the injected 5% dextrose solution may exert biochemical effects, such as alleviation of perineural glycopenia or modulation of pain via downregulation of nociceptive ion channels, including transient receptor potential vanilloid receptor-1 (TRPV1) [
13,
17,
32,
33]. We suggest that both mechanical and biochemical mechanisms may have contributed to the clinical improvement observed in our patient.
The targeted delivery of the injectate, facilitated by real-time ultrasound guidance, allowed for precise circumferential hydrodissection of the SN, ensuring complete release from surrounding tissues. This was confirmed intraoperatively by the visual observation of the injectate surrounding the nerve medially, laterally, anteriorly, and posteriorly. Ultrasound imaging is crucial for visualizing fascial abnormalities such as thickening, adhesions, and altered echotexture that may contribute to nerve entrapment. The real-time visualization allows for precise needle placement and targeted release of the nerve from these fascial constraints.
The use of SDP was crucial in both diagnosing and confirming the SN entrapment in this case. SDP allowed for precise localization of the point of maximal tenderness and reproduction of the patient’s concordant pain, providing a dynamic assessment of nerve involvement. This technique, combined with ultrasound imaging, offers a more comprehensive evaluation compared to static imaging alone, enabling the clinician to identify subtle nerve compressions that may be missed on standard ultrasound examination. The immediate post-hydrodissection SDP findings, which demonstrated improved nerve mobility and absence of pain reproduction, further validated the effectiveness of the intervention.
Psoriasis patients frequently exhibit enthesopathy, detectable via ultrasonography even without clinical symptoms, which may represent early indicators of psoriatic arthritis [
2]. Notably, recent studies indicate broader neurologic involvement in psoriasis, including peripheral neuropathy potentially linked to elevated inflammatory cytokines and immune dysregulation [
28]. In our patient, the presence of a psoriatic skin lesion proximal to the nerve entrapment site emphasizes a possible association between psoriasis-related inflammation and nerve compression. Although no overt inflammatory signs were evident on Doppler ultrasound, the nerve entrapment located beneath the psoriatic skin lesion suggests potential biochemical contributions alongside mechanical factors. Hydrodissection directly targets the fascia surrounding the nerve, mechanically separating the nerve from these compressive forces. The injected fluid not only creates space but may also help to break down adhesions and improve fascial mobility.
5. Limitations
Several limitations should be acknowledged. Firstly, as this report describes a single case, findings may not be directly generalizable to broader patient populations. While the positive outcome is encouraging, it is essential to recognize that individual patient responses can vary, and the efficacy of US-guided HD for sural nerve entrapment in the context of psoriasis-related Achilles tendinopathy needs to be validated in larger, more diverse cohorts.
Secondly, the absence of long-term ultrasonographic follow-up restricts objective evaluation of morphological changes and nerve recovery post-procedure. While the patient reported sustained symptom relief at 18 months, the lack of serial ultrasound imaging prevents us from assessing potential changes in nerve size, echotexture, or surrounding tissue architecture. Future studies should incorporate longitudinal ultrasound assessments to correlate clinical outcomes with objective measures of nerve health and structural changes. Further research could explore the use of quantitative ultrasound techniques, such as elastography, to assess fascial remodeling and changes in tissue stiffness following hydrodissection in similar cases. These techniques could provide objective measures of fascial health and treatment response.
Thirdly, the exact interaction of mechanical versus biochemical mechanisms underlying clinical improvement, particularly in the context of psoriasis, remains uncertain. Although we hypothesize that both mechanical separation of the nerve and potential anti-inflammatory effects of D5W contributed to the positive outcome, the relative contribution of each mechanism remains speculative. Further research, potentially involving in vitro studies or animal models, is needed to elucidate the specific molecular pathways involved.
Furthermore, the reliance on a single outcome measure (NPRS score) and subjective patient reports introduces potential bias. While the reduction in NPRS scores and the patient’s reported resolution of stiffness are valuable indicators, the absence of objective functional assessments (e.g., gait analysis, ankle range of motion measurements) limits the comprehensiveness of the evaluation. The lack of objective measures makes it difficult to definitively attribute the observed improvement solely to the intervention, as patient perception may be influenced by various factors. Future studies should incorporate a battery of objective outcome measures to provide a more robust assessment of functional improvement.
Finally, the lack of a control group makes it difficult to definitively attribute the observed improvement solely to the US-guided HD. While the patient had failed previous conservative treatments, the possibility of spontaneous resolution or placebo effects cannot be entirely ruled out. A randomized controlled trial comparing US-guided HD to a sham intervention or other established treatments would be necessary to establish the true efficacy of this approach.
Thus, further systematic studies with larger sample sizes, comprehensive follow-up (including both clinical and ultrasonographic assessments), and robust study designs (e.g., randomized controlled trials) are warranted to elucidate the exact mechanisms underlying US-guided HD’s clinical efficacy in psoriasis-associated neuropathy and to determine its optimal role in the management of sural nerve entrapment.
6. Conclusions
This case demonstrates the potential efficacy of US-guided HD, guided by SDP, in managing neuropathic Achilles tendon pain unresponsive to conventional treatment in a patient with psoriasis. SDP proved valuable in both diagnosing and confirming SN entrapment. To our knowledge, this is the first report of successful US-guided HD treatment of SN entrapment associated with a psoriatic skin lesion. The precise, circumferential hydrodissection of the SN, facilitated by real-time ultrasound guidance, appears to be a promising approach for releasing nerve compression and alleviating associated pain. This case also highlights the potential role of fascial pathology in contributing to nerve entrapment and the importance of considering fascial structures in the diagnosis and treatment of such conditions.
This case emphasizes neuropathic pain as a potentially overlooked complication in patients with psoriasis and highlights the importance of early diagnosis utilizing peripheral nerve ultrasonography, incorporating dynamic assessment techniques like SDP. However, given the limitations of a single case report, including the lack of a control group and the reliance on subjective outcome measures, these findings should be interpreted with caution.
Further research is warranted to elucidate the precise underlying mechanisms (including the relative contributions of mechanical and biochemical effects), determine the prevalence, and characterize the pathophysiological aspects of neuropathic pain in patients with psoriasis. Specifically, future studies should employ larger sample sizes, incorporate objective functional assessments and long-term ultrasonographic follow-up, and utilize robust study designs such as randomized controlled trials to systematically evaluate the long-term efficacy and clinical outcomes of US-guided HD for sural nerve entrapment. Future investigations should also focus on characterizing the fascial characteristics in nerve entrapment syndromes, potentially utilizing quantitative ultrasound techniques to assess fascial thickness, echogenicity, and elasticity.
Author Contributions
Conceptualization, YY and KHSL; methodology, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; software, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; validation, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; resources, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; writing—original draft preparation, HWL, YY and KHSL; writing—review and editing, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; visualization, YY and KHSL; supervision, YY, KDR and KHSL.; project administration, HWL, YY, CP, SK, ML, JL, JHH, DS, KDR, KHSL; funding acquisition, YY and KHSL.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the research involves the use of collections of information or data from which all personal identifiers have been removed prior to being received by the researchers.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data related to this study has been included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campanati, A. , et al., Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines, 2021. 9(11). [CrossRef]
- Fiorenza, A. , et al., Assessment of enthesis in patients with psoriatic arthritis and fibromyalgia using clinical examination and ultrasound. Clin Exp Rheumatol, 2020. 38 Suppl 123(1): p. 31-39.
- Spinnato, P. , et al., Imaging of Musculoskeletal Soft-Tissue Infections in Clinical Practice: A Comprehensive Updated Review. Microorganisms, 2022. 10(12). [CrossRef]
- Zaidman, C.M. , et al., Detection of peripheral nerve pathology: comparison of ultrasound and MRI. Neurology, 2013. 80(18): p. 1634-40.
- Hetland, M.L. , DANBIO--powerful research database and electronic patient record. Rheumatology (Oxford), 2011. 50(1): p. 69-77. [CrossRef]
- Rifbjerg-Madsen, S. , et al., Pain and pain mechanisms in patients with inflammatory arthritis: A Danish nationwide cross-sectional DANBIO registry survey. PLOS ONE, 2017. 12(7): p. e0180014. [CrossRef]
- Kılıçoğlu, M.S., M. Kara, and O.V. Yurdakul, Neuropathic Pain in Patients with Psoriatic Arthritis: A Bystander or a Gamechanger? Sakarya Tıp Dergisi, 2023. 13(3): p. 412-420.
- Bhuyan, D. and J.P. Tople, Ultrasound-guided Hydrodissection of Sural Nerve for Foot Pain. Journal of Pharmaceutical Research International, 2021: p. 513-518. [CrossRef]
- Bianchi, S. , et al., Ultrasonography of the sural nerve: normal and pathologic appearances. Journal of Ultrasound in Medicine, 2018. 37(5): p. 1257-1265. [CrossRef]
- Brown, M.N., B. S. Pearce, and T.K. Vanetti, Sural nerve entrapment. Peripheral nerve entrapments: clinical diagnosis and management, 2016: p. 795-810.
- Paraskevas, G.K. , et al., Fascial entrapment of the sural nerve and its clinical relevance. Anatomy & Cell Biology, 2014. 47(2): p. 144-147. [CrossRef]
- Hirose, C.B. and W.C. McGarvey, Peripheral nerve entrapments. Foot Ankle Clin, 2004. 9(2): p. 255-69.
- Lam, K.H.S. , et al., Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J Pain Res, 2020. 13: p. 1957-1968. [CrossRef]
- Omodani, T. and K. Takahashi, Ultrasound-Guided Hydrodissection for Sural Neuropathy After Calcaneus Fracture Surgery: A Case Report. Cureus, 2023. 15(10): p. e47749. [CrossRef]
- Tople, J. and D. Bhuyan, Ultrasound-guided hydrodissection of sural nerve for foot pain- A case report. Authorea, 2021.
- Fader, R.R. , et al., Percutaneous Ultrasound-Guided Hydrodissection of a Symptomatic Sural Neuroma. Orthopedics, 2015. 38(11): p. e1046-50. [CrossRef]
- Lam, K.H.S. , et al., Ultrasound-Guided Interventions for Carpal Tunnel Syndrome: A Systematic Review and Meta-Analyses. Diagnostics (Basel), 2023. 13(6). [CrossRef]
- Chan, O. , et al., High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil, 2008. 30(20-22): p. 1697-708. [CrossRef]
- Kakkos, G.A. , et al., US-guided high-volume injection for Achilles tendinopathy. J Ultrason, 2021. 21(85): p. e127-e133. [CrossRef]
- Nielsen, T.G. , et al., The effect of high-volume image-guided injection in the chronic non-insertional Achilles tendinopathy: a retrospective case series. J Exp Orthop, 2020. 7(1): p. 45. [CrossRef]
- Lam, K.H.S. , et al., Infraspinatus Fascial Dysfunction as a Cause of Painful Anterior Shoulder Snapping: Its Visualization via Dynamic Ultrasound and Its Resolution via Diagnostic Ultrasound-Guided Injection. Diagnostics (Basel), 2023. 13(15). [CrossRef]
- Lam, K.H.S. , et al., Novel Ultrasound-Guided Cervical Intervertebral Disc Injection of Platelet-Rich Plasma for Cervicodiscogenic Pain: A Case Report and Technical Note. Healthcare (Basel), 2022. 10(8). [CrossRef]
- Lam, K.H.S. , et al., Comment on the safety of the ultrasound-guided hydrodissection technique for carpal tunnel syndrome. Journal of Ultrasound, 2022. [CrossRef]
- Ozog, M.K. , et al., Neurological Complications of Biological Treatment of Psoriasis. Life (Basel), 2022. 12(1). [CrossRef]
- Otar Yener, G. ,Z. Ekici Tekin, and S. Yuksel, Psoriatic fasciitis in a pediatric patient: A case report. World J Clin Cases, 2019. 7(1): p. 69-72. [CrossRef]
- D’Onghia, M. , et al., Psoriasis and Fibromyalgia: A Systematic Review. J Pers Med, 2024. 14(2). [CrossRef]
- Erdem, C.Z. , et al., MR imaging features of foot involvement in patients with psoriasis. Eur J Radiol, 2008. 67(3): p. 521-5. [CrossRef]
- Kulakli, S. , et al., From a dermatologist point of view, enthesopathy and peripheral neuropathy in psoriasis patients. Archives of Current Medical Research, 2022. 3(3): p. 193-198. [CrossRef]
- Zhang, X. and Y. He, The Role of Nociceptive Neurons in the Pathogenesis of Psoriasis. Front Immunol, 2020. 11: p. 1984. [CrossRef]
- Barker-Davies, R.M. , et al., High-Volume Image-Guided Injections in Achilles and Patellar Tendinopathy in a Young Active Military Population: A Double-Blind Randomized Controlled Trial. Orthop J Sports Med, 2022. 10(4): p. 23259671221088326. [CrossRef]
- Watanabe, K. , et al., Ultrasound-Guided Hydrodissection of an Entrapped Saphenous Nerve After Lower Extremity Varicose Vein Stripping: A Case Report. A A Pract, 2020. 14(1): p. 28-30. [CrossRef]
- Lin, C.P. , et al., Utility of ultrasound elastography in evaluation of carpal tunnel syndrome: A systematic review and meta-analysis. Ultrasound Med Biol, 2019. 45(11): p. 2855-2865. [CrossRef]
- Wu, Y.T. , et al., Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol, 2018. 84(4): p. 601-610. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).