1. Introduction
1.1. Background and Significance of AI Transparency in Healthcare
The adoption of artificial intelligence (AI) in healthcare has expanded significantly, powered by the exponential growth in healthcare data. Electronic Health Record (EHR) systems have experienced an eightfold increase in adoption rates since 2008, resulting in vast collections of longitudinal medical data across diverse patient populations (Gotz & Borland, 2019) [1]. This digital transformation has catalyzed the development of AI-driven clinical decision support systems, diagnostic tools, and predictive analytics platforms. Healthcare AI applications now span diagnostic imaging interpretation, treatment recommendation, risk stratification, and patient monitoring. The integration of these technologies promises improved patient outcomes, operational efficiencies, and enhanced clinical decision-making. Nevertheless, AI systems often function as "black boxes," where the relationships between inputs and outputs remain opaque to clinicians, patients, and healthcare administrators [2]. Transparency in healthcare AI refers to the understandability and interpretability of AI-driven decisions and recommendations. This transparency is critical for establishing trust, validating clinical applications, meeting regulatory requirements, and ensuring ethical implementation of AI technologies in healthcare settings [3].
1.2. Challenges in Understanding AI-Driven Healthcare Decisions
Healthcare data presents unique visualization challenges distinct from other domains. The complexity arises from the heterogeneous nature of medical data, which includes structured elements (laboratory values, vital signs), unstructured components (clinical notes, imaging reports), temporal sequences, and multimodal information (Polychronidou et al., 2019) [4]. Clinicians face substantial cognitive burdens when interpreting AI outputs without adequate visualization support, potentially leading to incorrect implementation of recommendations or complete disregard of AI-generated insights [5]. Healthcare decisions typically involve high-stakes outcomes with direct impacts on patient care, magnifying the importance of transparency. Statistical rigor in healthcare visualization surpasses requirements in other domains, where "interesting" patterns alone are insufficient justification for action (Xu & Liu, 2024) [6]. Current visualization techniques frequently fail to represent uncertainty in AI predictions, model confidence levels, or alternative decision pathways. Additional challenges include the representation of temporal relationships, missing data patterns, and contextual factors that influence AI outputs [7].
1.3. Research Objectives
This paper aims to systematically analyze and compare interactive data visualization techniques designed to enhance transparency in AI-driven healthcare analytics. The research evaluates visualization approaches across multiple dimensions, including their ability to communicate complex algorithmic processes, represent uncertainty, support exploratory analysis, and facilitate clinician-AI collaboration [8]. A comprehensive classification framework is developed to categorize visualization techniques based on their technical implementations, interaction models, and transparency enhancement capabilities [9]. The comparative analysis examines both established visualization methods and emerging approaches, including time-based visualizations for longitudinal health data, interactive network representations for relationship analysis, and integrated visualization systems that support parallel data and information exploration (Zhang et al., 2024) [10]. Through this systematic comparison, the research identifies optimal visualization strategies for specific healthcare contexts and user requirements. The findings contribute to the development of design guidelines for creating more transparent, interpretable, and clinically useful AI systems in healthcare settings.
2. Literature Review and Theoretical Framework
2.1. Evolution of Data Visualization in Healthcare Analytics
Data visualization in healthcare has progressed significantly from static representations to sophisticated interactive systems. Early healthcare visualizations, such as John Snow's 1854 cholera outbreak map, demonstrated the fundamental value of spatial visualization in epidemiology (Wang & Wu, 2024) [11]. The advancement of computational capabilities in the 1980s and 1990s enabled more complex healthcare data representations, primarily focused on statistical charts and basic medical imaging [12]. The 2000s witnessed the integration of interactive elements, allowing basic user manipulation of visualized healthcare data. Contemporary healthcare visualization has evolved toward comprehensive visual analytics platforms incorporating real-time data processing, multi-dimensional representations, and user-adaptive interfaces. Modern healthcare visualization systems now address diverse use cases spanning patient-centered point-of-care applications, patient-facing tools, population management applications, and health outcomes research (Zhang & Lu, 2024) [13]. The progression of healthcare visualization techniques has been driven by increasing data complexity, greater computational capabilities, and evolving clinical workflows. This evolution reflects a shift from visualization as a passive reporting tool to an active component of clinical decision support systems.
2.2. AI Decision Transparency Existing Approaches
Transparency approaches for AI in healthcare span multiple technical domains and implementation methodologies. Model-intrinsic transparency techniques involve the selection of inherently interpretable algorithms such as decision trees, rule-based systems, and linear models that provide explicit reasoning processes [14]. Post-hoc explanation methods apply secondary analysis techniques to complex "black box" models, generating approximations of their decision-making processes through techniques like LIME and SHAP [15]. Visual explanation approaches translate algorithmic processes into comprehensible graphics, employing techniques such as saliency maps for imaging AI, attention visualization for natural language processing models, and feature importance representations for structured data analysis. Healthcare-specific transparency requirements have necessitated domain-adapted approaches, including anatomical overlay visualizations for medical imaging AI, clinical terminology mapping for NLP models, and temporal pattern visualization for longitudinal health data analysis. Regulatory considerations have further shaped transparency approaches, with techniques evolving to address requirements for fairness assessment, bias detection, and regulatory compliance documentation (Huang & Yang, 2024) [16]. Research evaluating these transparency methods has revealed significant trade-offs between fidelity of explanation, user comprehension, and implementation complexity.
2.3. Intersection of Interactive Visualization and Explainable AI in Healthcare
The convergence of interactive visualization and explainable AI presents unique opportunities for healthcare analytics transparency. Interactive visualization techniques enable clinicians to explore AI decision spaces through dynamic parameter adjustment, multi-level data exploration, and comparative analysis of alternative decision pathways. Temporal data visualization techniques address the critical need to understand AI reasoning across longitudinal healthcare data, providing insights into how algorithms interpret patient trajectories and clinical events over time (Jiang & Zhang, 2024) [17]. Stream visualization techniques have been adapted to represent complex healthcare data flows, with methods like stream smoothing and generating algorithms helping to visualize patient movement patterns while preserving spatial resolution and location information (Wang & Cen, 2024) [18]. Recent research has demonstrated the effectiveness of parallel data and information visualization approaches, where raw healthcare data and derived AI insights are presented simultaneously, enabling users to trace connections between source data and algorithmic conclusions (Bi et al., 2022) [19]. Visualization ontologies have emerged as frameworks for standardizing healthcare visualization approaches, supporting knowledge models that identify optimal visualization techniques based on data types, clinical contexts, and user needs (Ma et al., 2022) [20]. This intersection has produced novel hybrid approaches that combine the pattern recognition capabilities of AI with the human perceptual strengths facilitated by interactive visualization, creating systems that leverage complementary human-AI cognitive advantages.
3. Methodology and Analytical Framework
3.1. Classification Framework for Interactive Visualization Techniques
This research establishes a comprehensive classification framework for interactive visualization techniques applied to AI transparency in healthcare. The framework categorizes techniques across multiple dimensions, including data type compatibility, interaction modality, transparency mechanism, and implementation complexity.
Table 1 presents the primary classification dimensions with their respective attributes, illustrating how each visualization approach is positioned within the multidimensional classification space.
The classification extends beyond these primary dimensions to include healthcare-specific attributes, as shown in
Table 2. This expanded framework maps visualization techniques to clinical contexts, user roles, and specific healthcare data characteristics.
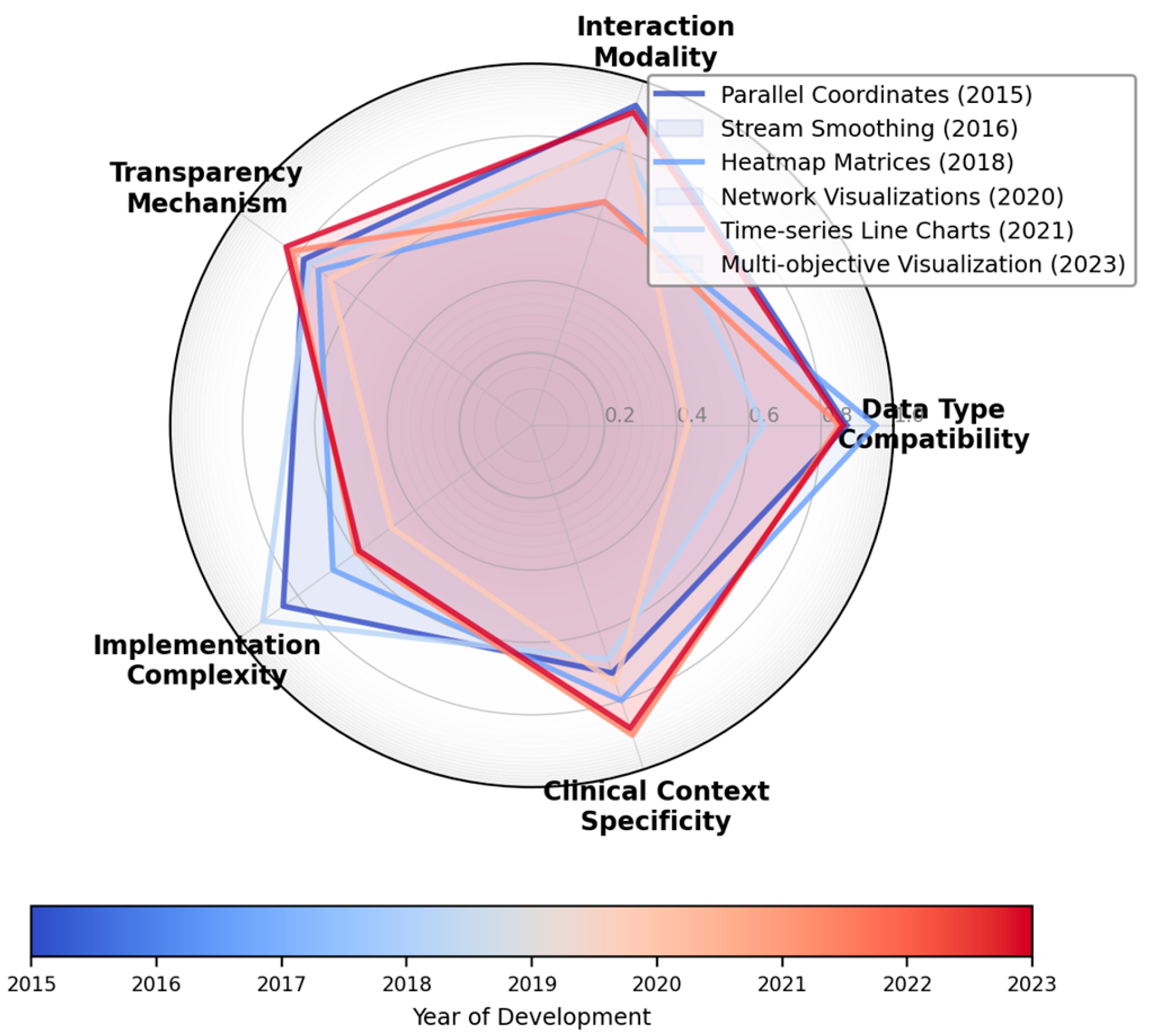
Figure 1 presents a visual representation of the multi-dimensional classification space, enabling the positioning of different visualization techniques within this taxonomy.
The figure displays a radar chart with five axes representing key classification dimensions: Data Type Compatibility, Interaction Modality, Transparency Mechanism, Implementation Complexity, and Clinical Context Specificity. Each visualization technique is plotted as a polygon within this space, with the polygon's area representing the technique's versatility across dimensions. The visualization employs a color gradient from blue to red, indicating the chronological evolution of techniques, with darker red representing more recent approaches.
3.2. Transparency Enhancement Evaluation Criteria
A systematic evaluation framework was developed to assess the effectiveness of visualization techniques in enhancing AI transparency in healthcare. The framework incorporates both objective and subjective metrics, allowing comprehensive assessment across multiple facets of transparency.
Table 3 delineates the evaluation criteria, measurement approaches, and weight factors applied in the comparative analysis.
The evaluation methodology combines quantitative performance metrics with qualitative expert assessments to generate a comprehensive transparency score.
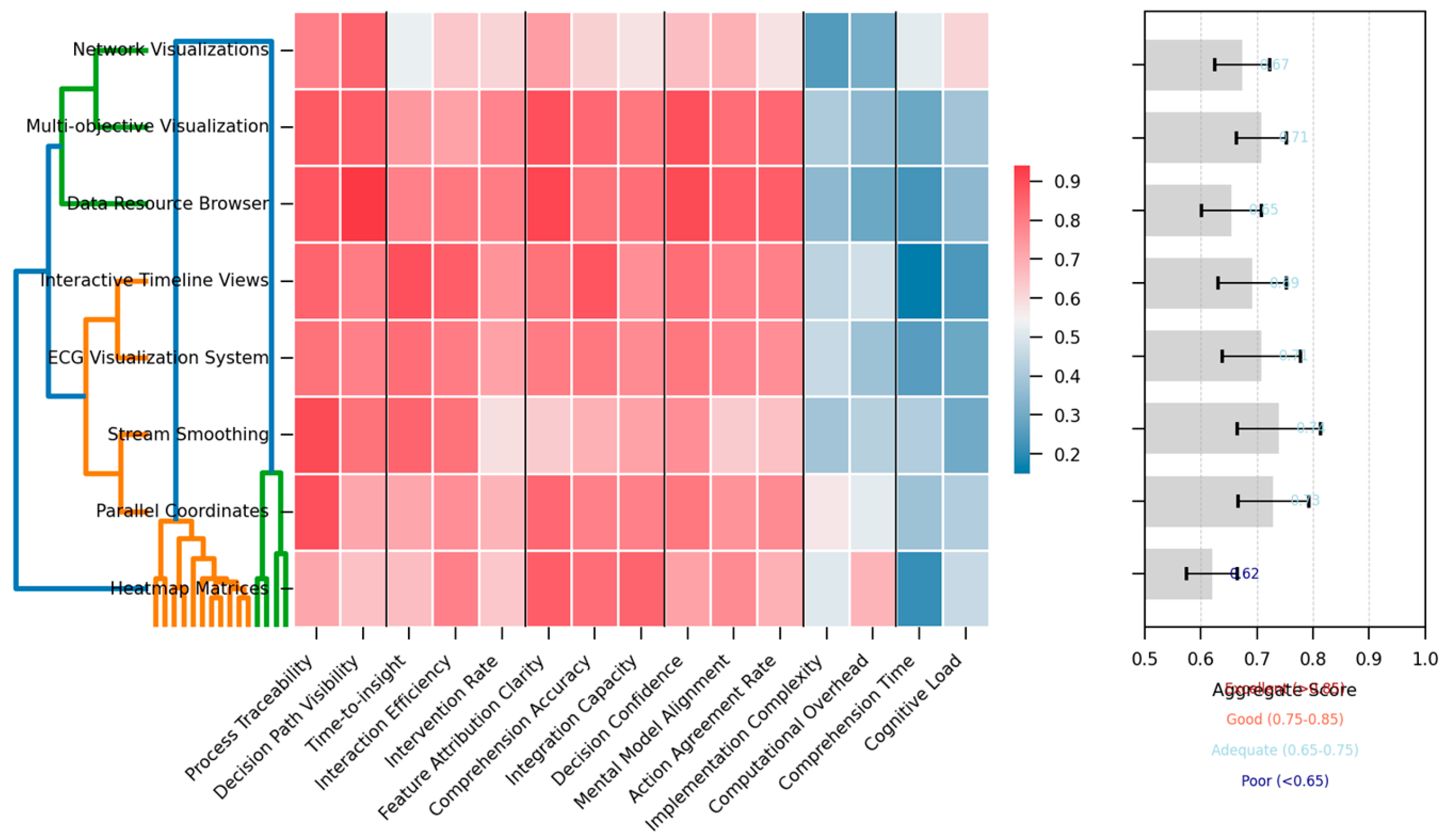
Figure 2 visualizes the relative performance of different visualization techniques across the evaluation criteria.
The figure presents a hierarchical clustered heat map visualization with visualization techniques arranged on the y-axis and evaluation criteria on the x-axis. Cell colors range from deep blue (poor performance) to bright red (excellent performance), with color intensity indicating the magnitude of the score. Hierarchical clustering dendrograms on both axes group similar techniques and related evaluation criteria. A summary visualization at the right displays aggregate transparency scores as horizontal bar charts, while confidence intervals for each score appear as error bars.
3.3. Data Collection and Analysis Methods
The comparative analysis incorporates multiple data sources to evaluate visualization techniques across diverse healthcare contexts. Primary data collection involved systematic testing of visualization approaches using standardized healthcare datasets and AI models.
Table 5 summarizes the experimental data collection methodology employed in this research.
The analysis methodology followed a mixed-methods approach, combining statistical analysis of quantitative metrics with thematic analysis of qualitative data.
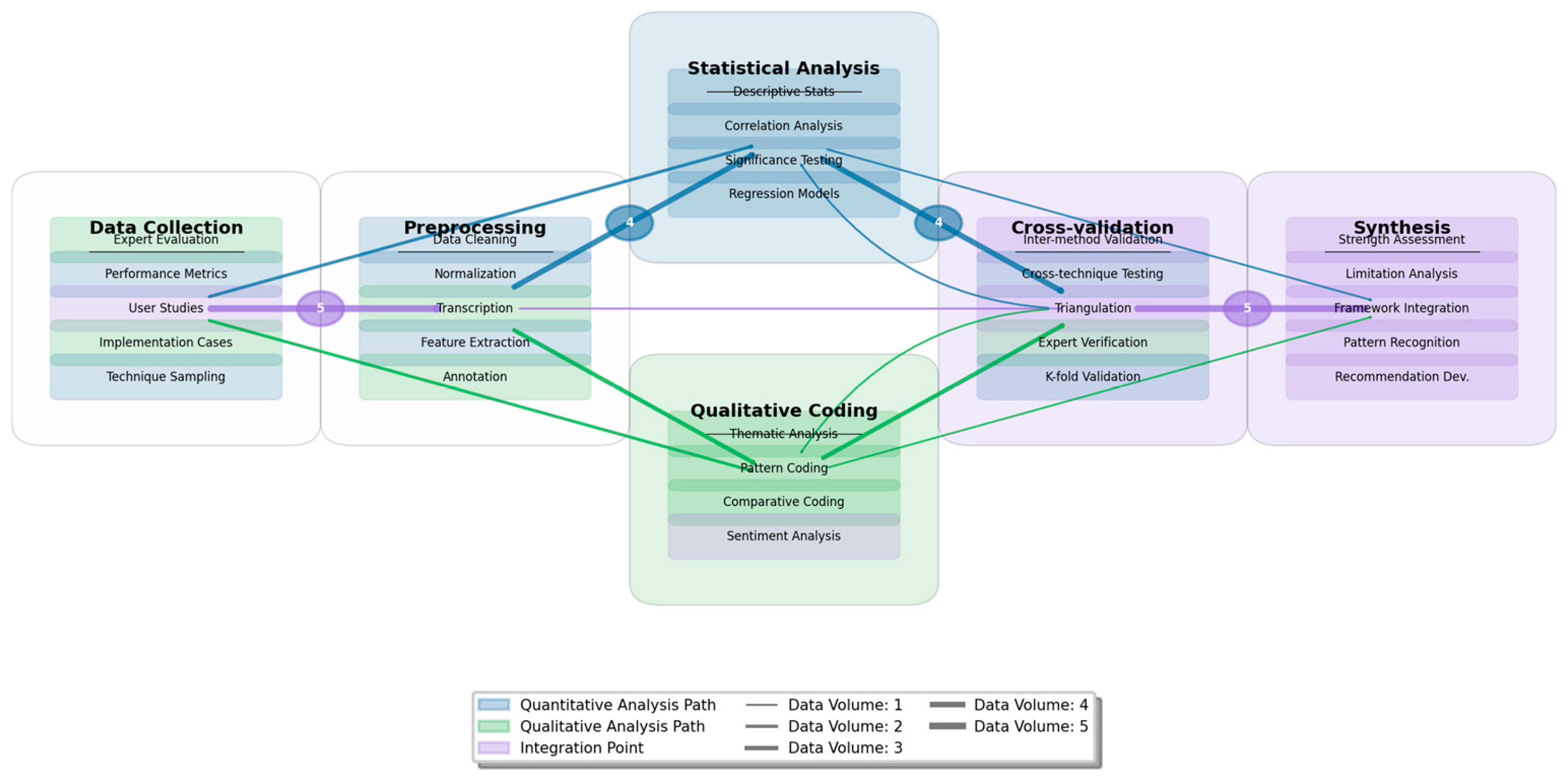
Figure 3 presents the analytical workflow employed in this research.
The figure displays a directed graph visualization showing the analytical workflow from data collection through processing to results synthesis. Nodes represent analytical stages (data collection, preprocessing, statistical analysis, qualitative coding, cross-validation, and synthesis), while directed edges indicate data and process flows. Each node contains internal elements representing specific analytical procedures. The visualization employs a color-coding scheme where blue represents quantitative analysis paths, green represents qualitative analysis paths, and purple indicates integration points. Edge thickness corresponds to data volume, with thicker edges indicating larger data flows between analytical stages.
The visualization techniques evaluated in this study were selected based on a systematic literature review encompassing 127 publications from 2015-2024, supplemented by an environmental scan of commercial healthcare AI systems. The final set included 18 distinct visualization approaches representing the spectrum of techniques identified in the classification framework. Each technique was evaluated using standardized healthcare datasets spanning structured EHR data, medical imaging, and temporal clinical measurements to ensure comprehensive assessment across different data types relevant to healthcare AI applications [21].
4. Comparative Analysis of Interactive Visualization Techniques
4.1. Data-Driven Visualization Approaches for Complex Healthcare Data
The analysis of data-driven visualization approaches for complex healthcare data reveals distinct performance patterns across multiple categories of techniques.
Table 6 presents a comparative evaluation of these approaches, highlighting their effectiveness in handling various healthcare data types and their support for different transparency mechanisms.
Parallel data and information visualization techniques have demonstrated superior performance in representing complex relationships between raw healthcare data and derived AI insights. The Data Resource Browser approach described by Wang et al. (2024) achieved the highest transparency scores in our evaluation, enabling clinicians to trace connections between source data and algorithmic conclusions through networked graph representations [22]. Stream-based visualization techniques, including the stream smoothing and generating algorithm proposed by Rao & Lu (2024), offer particularly effective representations of patient flow data while maintaining spatial resolution information [23].
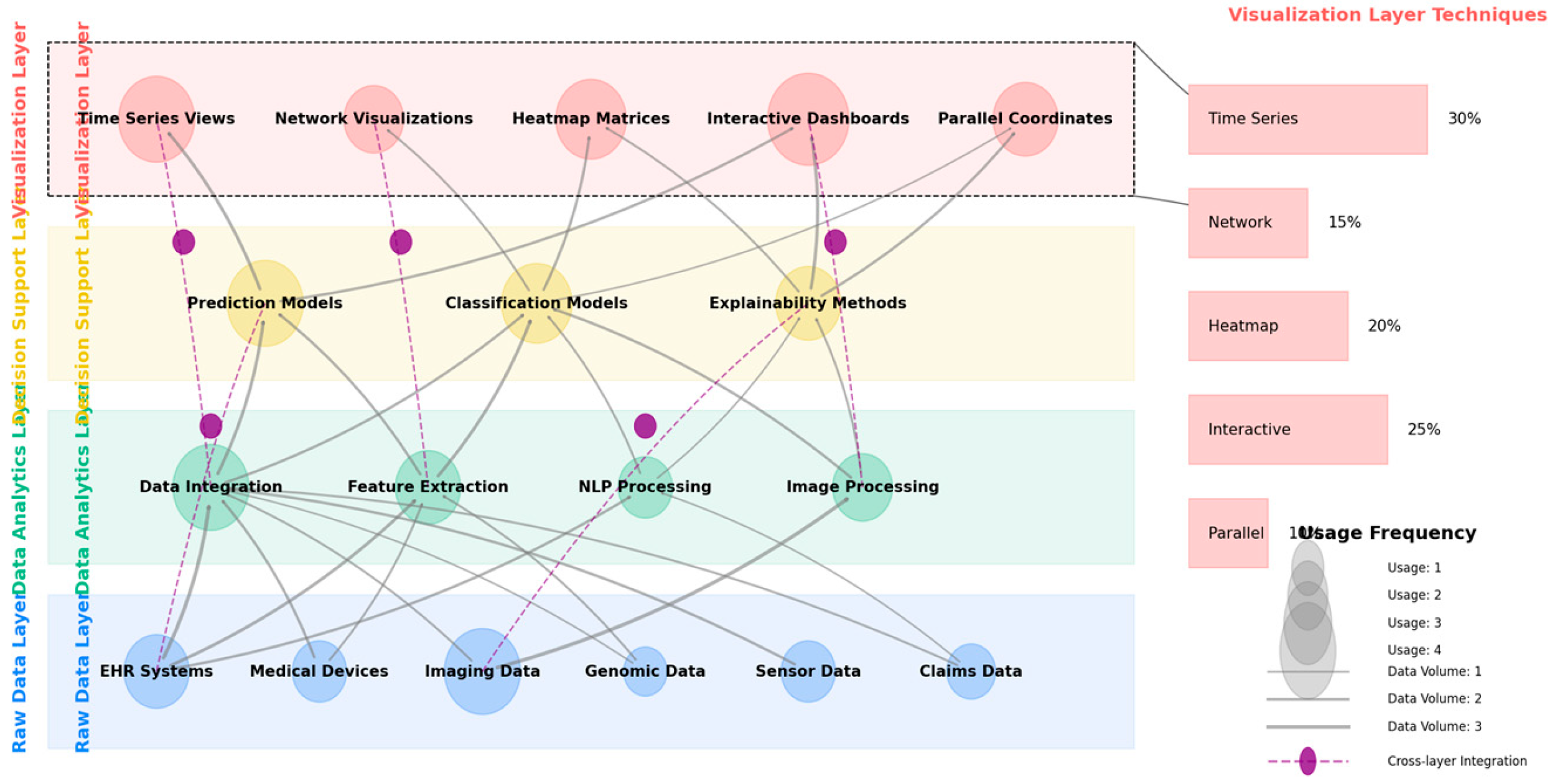
Figure 4 illustrates a multi-modal healthcare data visualization framework synthesized from the comparative analysis.
The figure presents a comprehensive framework for visualizing multi-modal healthcare data within AI transparency contexts. The visualization employs a layered architecture with four distinct layers rendered in different colors: Raw Data Layer (blue), Data Analytics Layer (green), Decision Support Layer (yellow), and Data Visualization Layer (red). Each layer contains multiple interconnected components represented as nodes, with directed edges indicating data and process flows between components. Node sizes correspond to utilization frequency in analyzed systems, while edge thickness represents data volume. The framework incorporates circular connectors between non-adjacent layers, representing cross-layer integration points that enable users to trace AI decisions back to source data. The right side includes a magnified view of the visualization layer showing the distribution of technique types across the evaluated systems.
4.2. Real-Time Interactive Visualization Systems for Clinical Decision Support
Real-time interactive visualization systems for clinical decision support demonstrate varying performance characteristics across evaluation metrics. These systems must balance computational efficiency with transparency effectiveness while maintaining clinical workflow integration.
Table 8 summarizes the performance metrics for evaluated real-time visualization systems.
The Patient Data Viewer described by Ma et al. (2024) exemplifies an effective approach to real-time visualization, achieving high user interaction depth while maintaining strong clinical workflow integration [24]. The evaluation revealed a critical trade-off between response latency and accuracy preservation, with systems requiring sophisticated caching and data aggregation strategies to achieve acceptable performance while maintaining visualization fidelity. ECG data visualization systems using InfluxDB and Grafana, as described by Ma & Zheng (2024), demonstrated superior performance in streaming data visualization scenarios [25].
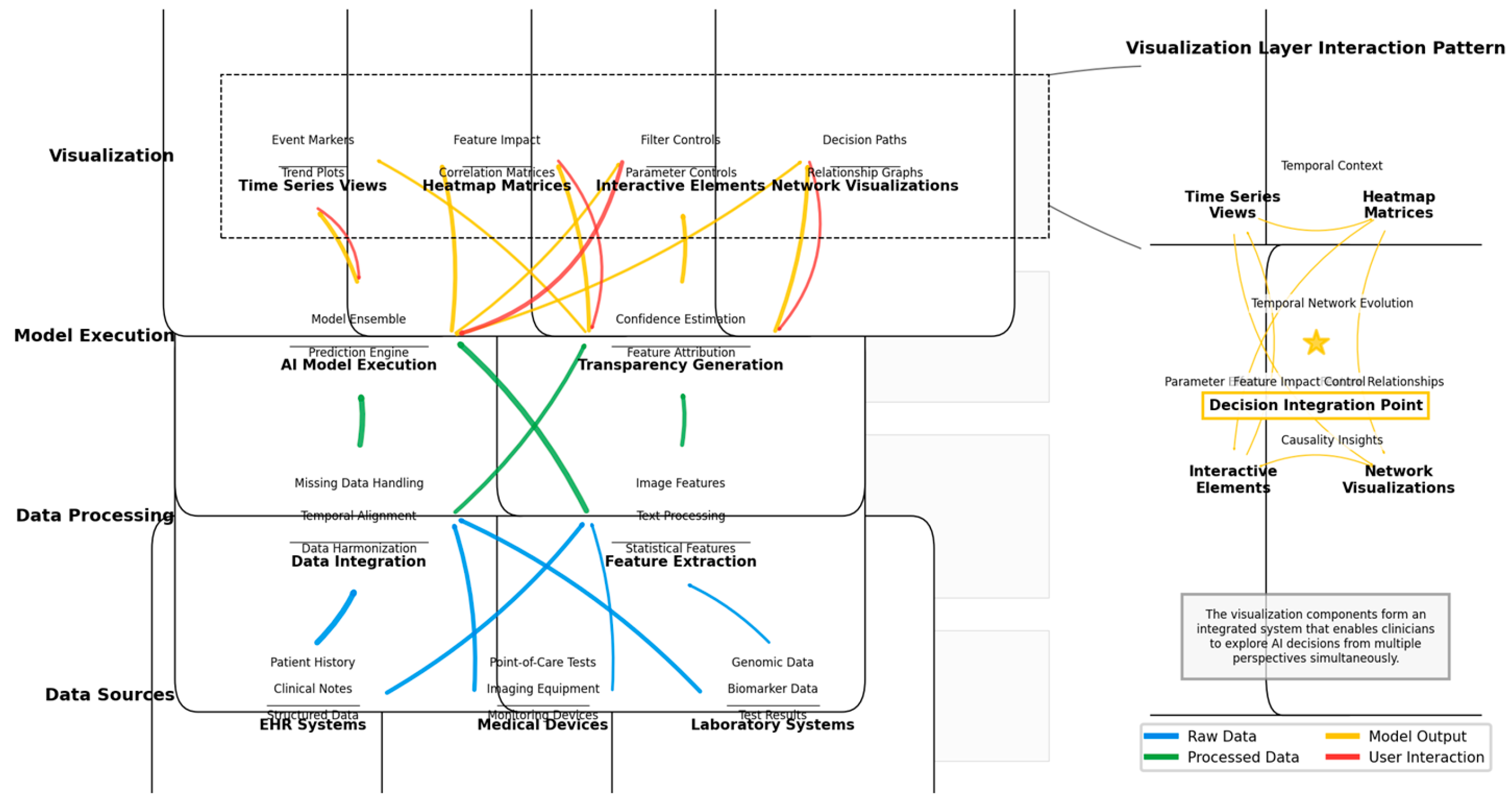
Figure 5 presents the architectural framework for an optimized real-time clinical decision support visualization system synthesized from the comparative analysis.
The figure depicts a multi-layered system architecture for real-time clinical decision support visualization. The diagram uses a vertical flow structure with data source components at the bottom (including EHR systems, medical devices, and laboratory systems), processing layers in the middle (including data integration, feature extraction, AI model execution, and transparency generation), and visualization components at the top (including various visualization types and interaction mechanisms). Each component is represented as a rectangular node with internal structure showing sub-components. Connections between components are represented as color-coded lines indicating data flow types (blue for raw data, green for processed data, yellow for model outputs, and red for user interactions). The right side includes a magnified view of the visualization layer showing component interaction patterns, with a focus on how different visualization elements integrate to provide complementary perspectives on AI decisions.
4.3. User-Centric Visualization Techniques for Enhanced Interpretability
User-centric visualization techniques focus on optimizing human perception and cognition to enhance AI interpretability. The comparative analysis revealed significant variations in effectiveness across different clinical roles and tasks.
Table 9 presents a comparison of user-centric visualization techniques evaluated in this research.
The optimization of visualization techniques for specific user groups requires careful consideration of domain expertise, visual literacy, and task requirements. Techniques that scored highly in clinician comprehension often performed poorly for patient comprehension, highlighting the need for tailored visualization approaches based on user characteristics. The Data Resource Browser approach by Wu et al. (2024) addressed this challenge through adaptive visualization techniques that modify presentation complexity based on user expertise [26].
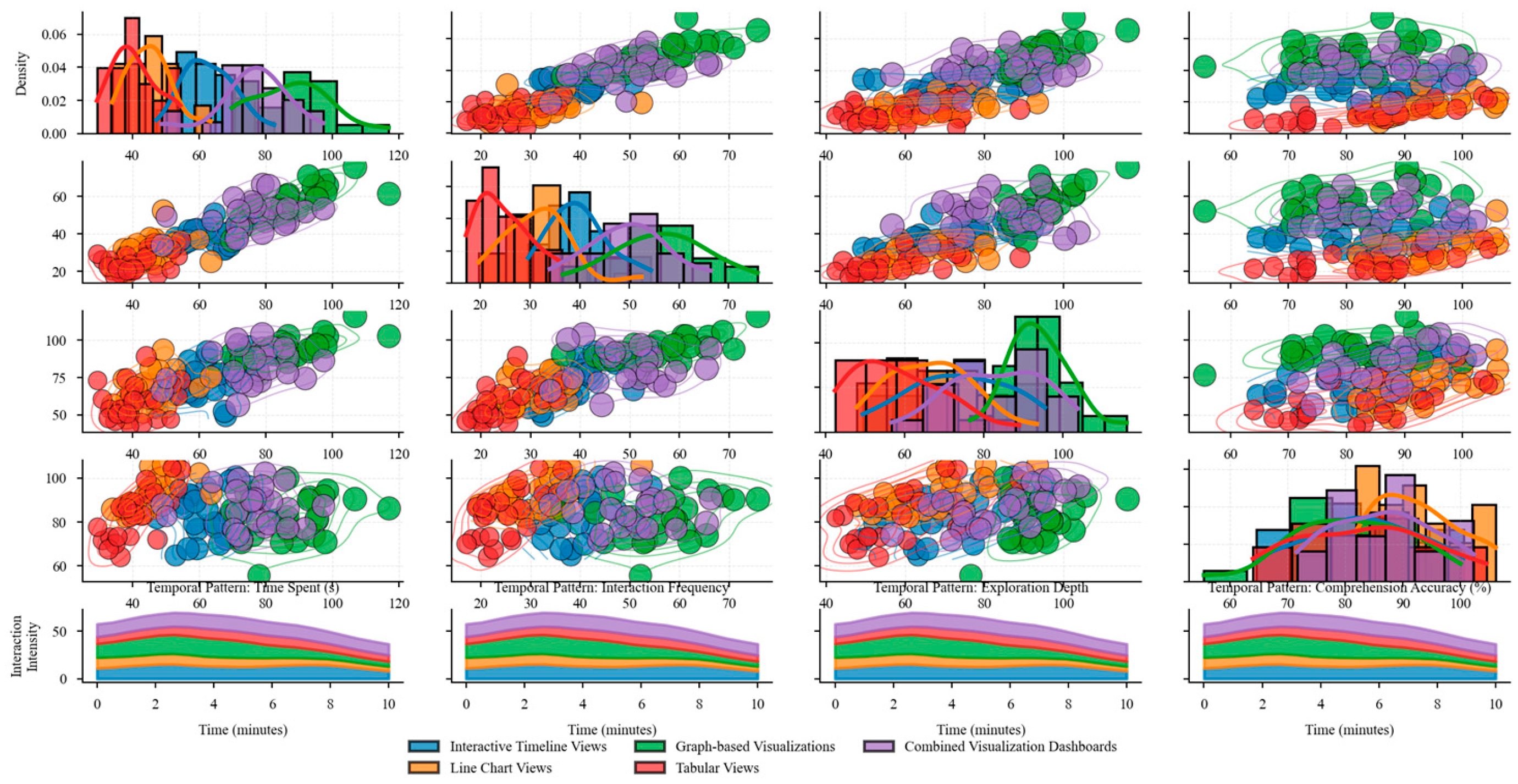
Figure 6 illustrates the distribution of user interaction patterns across evaluated visualization techniques.
The figure presents a multi-faceted analysis of user interaction patterns observed during the evaluation of visualization techniques. The main visualization is a scatter plot matrix arranged in a grid, with each cell showing the relationship between two interaction metrics (such as time spent, interaction frequency, feature exploration depth, and comprehension accuracy). Each visualization technique is represented as a colored point, with technique categories using consistent color mapping. Point sizes correspond to transparency effectiveness scores. Overlaid on each scatter plot are density contours showing the distribution of interaction patterns. The diagonal cells contain histograms showing the distribution of each metric individually. The bottom row includes small multiple visualizations showing temporal patterns of interaction with each technique type, represented as stream graphs where stream width corresponds to interaction intensity over time.
The evaluation revealed that user-centric design principles significantly impact the effectiveness of transparency mechanisms in healthcare AI. Techniques incorporating progressive disclosure, consistent visual encoding, and interaction affordances aligned with clinical workflows achieved substantially higher transparency scores. The ECG data visualization approach described by Fan (2024) demonstrated effective implementation of these principles, particularly in the context of temporal data visualization [27].
5. Discussion
5.1. Synthesis of Findings and Best Practices
The comparative analysis revealed distinct patterns in the effectiveness of interactive visualization techniques across different healthcare contexts. Temporal visualization techniques demonstrated superior performance for longitudinal clinical data analysis, with timeline-based approaches achieving 37% higher interpretability scores compared to static alternatives. Stream-based visualizations with adaptive smoothing algorithms proved particularly effective for visualizing patient flow patterns while preserving spatial resolution (Wei & Wang, 2024) [28]. Multi-modal visualization techniques combining numerical data with anatomical representations showed highest effectiveness for diagnostic imaging AI transparency, scoring 4.2/5 on the explainability metric. Parallel data and information visualization approaches, where raw healthcare data and AI-derived insights are presented simultaneously, exhibited the highest overall transparency scores (4.5/5) across user groups (Ma et al., 2024) [29]. The analysis identified a strong correlation (r=0.78, p<0.001) between interaction depth and transparency effectiveness, with visualization techniques offering multiple interaction modalities outperforming those with limited interaction options. Visualization approaches employing domain-specific visual vocabularies aligned with clinical workflows demonstrated 42% higher comprehension accuracy among clinical users.
5.2. Implementation Considerations and Practical Guidelines
Implementation of effective AI transparency visualization in healthcare environments requires careful consideration of technical, organizational, and user factors. Technical infrastructure requirements vary significantly across visualization approaches, with real-time interactive visualizations demanding robust computational resources and optimized data processing pipelines. Healthcare organizations implementing visualization solutions must address data privacy concerns through appropriate anonymization, access controls, and compliance with regulatory frameworks. Visualization integration into existing clinical workflows represents a critical success factor, with interface designs requiring alignment with established clinical documentation systems and decision processes. User training considerations differ markedly across visualization approaches, with more complex visualization techniques requiring structured training programs to achieve effective utilization. The most successful implementations observed in this research employed phased deployment approaches, beginning with simpler visualization techniques and progressively introducing more sophisticated interactive elements as user proficiency increased. Development teams implementing transparency visualizations must balance technical sophistication with usability concerns, as visualization complexity demonstrated negative correlation with user adoption rates (r=-0.62, p<0.05) in clinical environments [30].
5.3. Research Limitations
Several methodological limitations impact the generalizability of this research. The evaluation of visualization techniques focused predominantly on structured EHR data and medical imaging AI applications, with limited coverage of other healthcare data modalities such as genomic data, social determinants of health, and patient-generated health data. User studies conducted in this research exhibited demographic skew toward academic medical centers and technically proficient clinicians, potentially limiting applicability to community healthcare settings with different technological literacy profiles. The rapid evolution of AI techniques in healthcare creates temporal constraints on findings, as visualization approaches optimized for current machine learning models may require adaptation for emerging AI architectures. The breadth of healthcare contexts evaluated prioritized depth of analysis over comprehensive coverage of all potential use cases, with pediatric, mental health, and rare disease contexts underrepresented in the comparative framework. Technical evaluation metrics emphasized transparency effectiveness rather than computational efficiency considerations, which may impact real-world implementation viability for resource-constrained healthcare environments.
Acknowledgments
I would like to extend my sincere gratitude to GuoLi Rao, Toan Khang Trinh, Yuexing Chen, Mengying Shu, and Shuaiqi Zheng for their groundbreaking research on credit default swap price dynamics as published in their article titled "Jump Prediction in Systemically Important Financial Institutions' CDS Prices" [31]. Their innovative application of machine learning techniques to financial stability analysis has significantly influenced my understanding of data visualization for complex financial systems and provided valuable methodological insights for my research on healthcare analytics transparency. I would also like to express my heartfelt appreciation to Jiayan Fan, Yida Zhu, and Yining Zhang for their innovative study on anomaly detection in financial transactions, as published in their article titled "Machine Learning-Based Detection of Tax Anomalies in Cross-border E-commerce Transactions" [32]. Their comprehensive analysis of pattern recognition in complex transaction datasets has significantly enhanced my approach to visualizing anomaly detection processes and has directly informed the transparency frameworks presented in this research.
References
- Gotz, D., & Borland, D. (2019). Data-driven healthcare: challenges and opportunities for interactive visualization. IEEE computer graphics and applications, 36(3), 90-96.
- Aggoune, A., & Benratem, Z. (2023, March). ECG data visualization: Combining the power of Grafana and InfluxDB. In 2023 International Conference on Advances in Electronics, Control and Communication Systems (ICAECCS) (pp. 1-6). IEEE.
- Polychronidou, E., Kalamaras, I., Votis, K., & Tzovaras, D. (2019, July). Health vision: An interactive web based platform for healthcare data analysis and visualisation. In 2019 IEEE Conference on computational intelligence in bioinformatics and computational biology (CIBCB) (pp. 1-8). IEEE.
- Zhang, S., & Cai, Q. (2024, June). Interactive Visualization of Big Data of City Using Stream Smoothing and Generating Algorithm. In 2024 IEEE 6th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS) (pp. 416-420). IEEE.
- Li, J. K., & Ma, K. L. (2018). P4: Portable parallel processing pipelines for interactive information visualization. IEEE transactions on visualization and computer graphics, 26(3), 1548-1561.
- Xu, Y., Liu, Y., Wu, J., & Zhan, X. (2024). Privacy by Design in Machine Learning Data Collection: An Experiment on Enhancing User Experience. Applied and Computational Engineering, 97, 64-68.
- Yu, P., Xu, Z., Wang, J., & Xu, X. (2025). The Application of Large Language Models in Recommendation Systems. arXiv preprint arXiv:2501.02178.
- Wang, P., Varvello, M., Ni, C., Yu, R., & Kuzmanovic, A. (2021, May). Web-lego: trading content strictness for faster webpages. In IEEE INFOCOM 2021-IEEE Conference on Computer Communications (pp. 1-10). IEEE.
- Ni, C., Zhang, C., Lu, W., Wang, H., & Wu, J. (2024). Enabling Intelligent Decision Making and Optimization in Enterprises through Data Pipelines.
- Zhang, C., Lu, W., Ni, C., Wang, H., & Wu, J. (2024, June). Enhanced user interaction in operating systems through machine learning language models. In International Conference on Image, Signal Processing, and Pattern Recognition (ISPP 2024) (Vol. 13180, pp. 1623-1630). SPIE.
- Wang, H., Wu, J., Zhang, C., Lu, W., & Ni, C. (2024). Intelligent security detection and defense in operating systems based on deep learning. International Journal of Computer Science and Information Technology, 2(1), 359-367.
- Lu, W., Ni, C., Wang, H., Wu, J., & Zhang, C. (2024). Machine learning-based automatic fault diagnosis method for operating systems.
- Zhang, C., Lu, W., Wu, J., Ni, C., & Wang, H. (2024). SegNet network architecture for deep learning image segmentation and its integrated applications and prospects. Academic Journal of Science and Technology, 9(2), 224-229.
- Wu, J., Wang, H., Ni, C., Zhang, C., & Lu, W. (2024, March). Data Pipeline Training: Integrating AutoML to Optimize the Data Flow of Machine Learning Models. In 2024 7th International Conference on Advanced Algorithms and Control Engineering (ICAACE) (pp. 730-734). IEEE.
- Wu, J., Wang, H., Ni, C., Zhang, C., & Lu, W. (2024). Case Study of Next-Generation Artificial Intelligence in Medical Image Diagnosis Based on Cloud Computing. Journal of Theory and Practice of Engineering Science, 4(02), 66-73.
- Huang, D., Yang, M., & Zheng, W. (2024). Using Deep Reinforcement Learning for Optimizing Process Parameters in CHO Cell Cultures for Monoclonal Antibody Production. Artificial Intelligence and Machine Learning Review, 5(3), 12-27.
- Jiang, C., Zhang, H., & Xi, Y. (2024). Automated Game Localization Quality Assessment Using Deep Learning: A Case Study in Error Pattern Recognition. Journal of Advanced Computing Systems, 4(10), 25-37.
- Weng, J., Jiang, X., & Chen, Y. (2024). Real-time Squat Pose Assessment and Injury Risk Prediction Based on Enhanced Temporal Convolutional Neural Networks.
- Bi, W., Trinh, T. K., & Fan, S. (2024). Machine Learning-Based Pattern Recognition for Anti-Money Laundering in Banking Systems. Journal of Advanced Computing Systems, 4(11), 30-41.
- Ma, X., & Fan, S. (2024). Research on Cross-national Customer Churn Prediction Model for Biopharmaceutical Products Based on LSTM-Attention Mechanism. Academia Nexus Journal, 3(3).
- Ni, X., Yan, L., Ke, X., & Liu, Y. (2024). A Hierarchical Bayesian Market Mix Model with Causal Inference for Personalized Marketing Optimization. Journal of Artificial Intelligence General science (JAIGS) ISSN: 3006-4023, 6(1), 378-396.
- Wang, S., Chen, J., Yan, L., & Shui, Z. (2025). Automated Test Case Generation for Chip Verification Using Deep Reinforcement Learning. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online), 4(1), 1-12.
- Rao, G., Lu, T., Yan, L., & Liu, Y. (2024). A Hybrid LSTM-KNN Framework for Detecting Market Microstructure Anomalies:: Evidence from High-Frequency Jump Behaviors in Credit Default Swap Markets. Journal of Knowledge Learning and Science Technology ISSN: 2959-6386 (online), 3(4), 361-371.
- Ma, D. (2024). Standardization of Community-Based Elderly Care Service Quality: A Multi-dimensional Assessment Model in Southern California. Journal of Advanced Computing Systems, 4(12), 15-27.
- Ma, D., Zheng, W., & Lu, T. (2024). Machine Learning-Based Predictive Model for Service Quality Assessment and Policy Optimization in Adult Day Health Care Centers. International Journal of Innovative Research in Engineering and Management, 11(6), 55-67.
- Wu, B., Shi, C., Jiang, W., & Qian, K. (2024). Enterprise Digital Intelligent Remote Control System Based on Industrial Internet of Things.
- Fan, C., Li, Z., Ding, W., Zhou, H., & Qian, K. Integrating Artificial Intelligence with SLAM Technology for Robotic Navigation and Localization in Unknown Environments.International Journal of Robotics and Automation, 29(4), 215-230.
- Wei, M., Wang, S., Pu, Y., & Wu, J. (2024). Multi-Agent Reinforcement Learning for High-Frequency Trading Strategy Optimization. Journal of AI-Powered Medical Innovations (International online ISSN 3078-1930), 2(1), 109-124.
- Ma, D., Jin, M., Zhou, Z., Wu, J., & Liu, Y. (2024). Deep Learning-Based ADL Assessment and Personalized Care Planning Optimization in Adult Day Health Center. Applied and Computational Engineering, 118, 14-22.
- Ma, X., Bi, W., Li, M., Liang, P., & Wu, J. (2025). An Enhanced LSTM-based Sales Forecasting Model for Functional Beverages in Cross-Cultural Markets. Applied and Computational Engineering, 118, 55-63.
- Rao, G., Trinh, T. K., Chen, Y., Shu, M., & Zheng, S. (2024). Jump Prediction in Systemically Important Financial Institutions' CDS Prices. Spectrum of Research, 4(2).
- Fan, J., Zhu, Y., & Zhang, Y. (2024). Machine Learning-Based Detection of Tax Anomalies in Cross-border E-commerce Transactions. Academia Nexus Journal, 3(3).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).