Submitted:
18 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
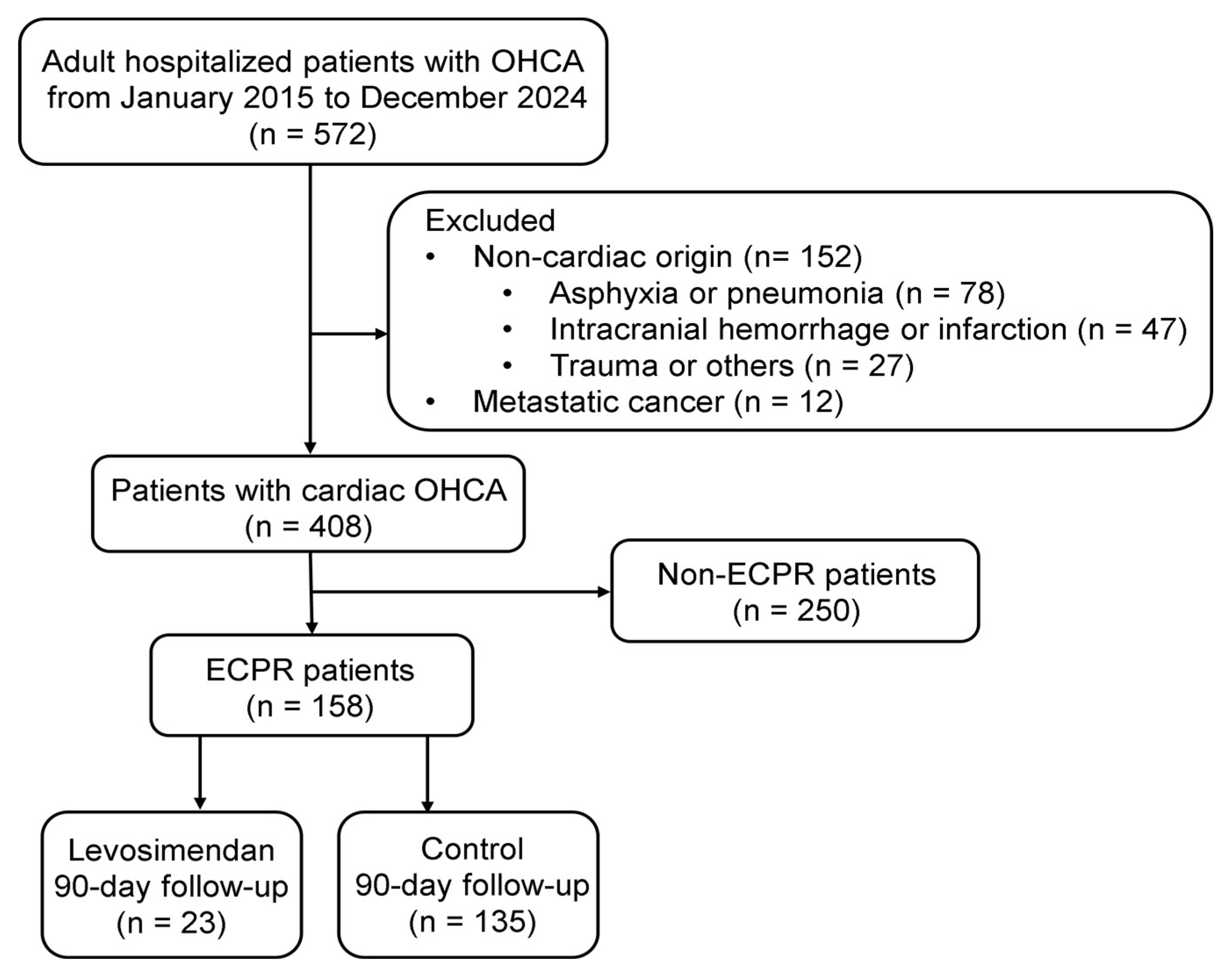
2.1. Study Design and Population
2.2. Variables, Data, Sources, and Measurements
2.3. Vasopressors (Epinephrine, Norepinephrine, and Vasopressin)
2.4. Inotropes (Dopamine and Dobutamine)
2.5. Inodilators (Milrinone and Levosimendan)
2.6. First 24-h VIS
2.7. Left Ventricular Function Test at 24 h After OHCA
2.8. Levosimendan Use Within 72 h of ECPR
2.9. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
|
Levosimendan (n = 23) |
Control (n = 135) |
p-value | ||
| Age (y), mean± SD | 54.1 ± 12.9 | 54.3 ± 14.1 | 0.96 | |
| Male sex, n (%) | 21 (91.3) | 117 (86.7) | 0.54 | |
| BMI (kg/m2), mean ± SD | 27.7 ± 4.1 | 26.8 ± 4.8 | 0.34 | |
|
Medical history, n (%) |
Hypertension | 13 (56.5) | 52 (38.5) | 0.11 |
| Hyperlipidemia | 9 (39.1) | 49 (36.3) | 0.66 | |
| Diabetes mellitus | 5 (21.7) | 28 (20.7) | 0.91 | |
| Chronic heart failure | 4 (17.4) | 20 (14.8) | 0.75 | |
| Coronary artery disease | 4 (17.4) | 26 (19.3) | 0.83 | |
| End-stage renal disease | 1 (4.3) | 7 (5.2) | 0.87 | |
| Cerebrovascular disease | 1 (4.3) | 3 (2.2) | 0.55 | |
| Chronic obstructive pulmonary disease | 1 (4.3) | 1 (0.7) | 0.15 | |
| Witnessed cardiac arrest, n (%) | 17 (74.3) | 92 (68.2) | 0.48 | |
| Bystander CPR, n (%) | 12 (52.2) | 57 (42.2) | 0.29 | |
| Initial shockable rhythm, n (%) | 2 (91.3) | 98 (72.6) | 0.05 | |
| No-flow time (min), mean ±SD | 4.6 ± 1.5 | 4.9 ± 0.4 | 0.51 | |
| CPR duration (min), mean± SD | 30.7 ± 13.7 | 35.5 ± 25.3 | 0.37 | |
| Initial arterial pH,mean ± SD | 7.04 ± 0.16 | 7.01 ± 0.19 | 0.39 | |
| CAHP score, mean ± SD | 140.3 ± 26.7 | 153.8 ± 7.1 | 0.13 | |
|
CHD, n (%) |
Coronary angiography | 15 (65.2) | 92 (68.1) | 0.78 |
| Left main disease | 6 (26.1) | 17 (12.6) | 0.09 | |
| Triple-vessel disease | 9 (39.1) | 47 (34.8) | 0.69 | |
|
AMI, n (%) |
Percutaneous coronary intervention | 14 (60.9) | 85 (63.0) | 0.85 |
| ST-elevation myocardial infarction | 12 (52.2) | 55 (40.7) | 0.31 | |
| Intra-aortic balloon pump, n (%) | 11 (47.8) | 38 (28.1) | 0.06 | |
| Targeted temperature management, n (%) | 17 (73.9) | 96 (71.1) | 0.78 | |
| Cardiogenic shock, n (%) | 22 (95.7) | 129 (95.6) | 0.98 | |
| SCAI Stage C, n (%) | 2 (8.7) | 29 (21.5) | ||
| SCAI Stage D, n (%) | 9 (39.1) | 37 (27.4) | ||
| SCAI Stage E, n (%) | 11 (47.8) | 63 (46.7) | ||
| The first 24-h VIS, mean ± SD | 235.7 ± 128.0 | 245.9 ± 178.6 | 0.79 | |
| Vasopressin (I/kg/min), mean ± SD | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Norepinephrine (μg/kg/min), mean ± SD | 0.3 ± 0.2 | 0.2 ± 0.2 | ||
| Epinephrine (μg/kg/min), mean ± SD | 2.0 ± 1.3 | 2.1 ± 1.7 | ||
| Dopamine (μg/kg/min), mean ± SD | 6.3 ± 7.7 | 8.1 ± 9.3 | ||
| Dobutamine (μg/kg/min), mean ± SD | 0.5 ± 1.5 | 0.2 ± 0.9 | ||
| LVEF at 24 h (%), mean ± SD | 31.2 ± 12.2 | 31.0 ± 15.4 | 0.97 | |
| LVCI at 24 h (L/min/M2), mean ± SD | 1.73 ± 0.83 | 1.60 ± 1.02 | 0.56 | |
| CPO at 24 h (W), mean ± SD | 0.54 ± 0.26 | 0.48 ± 0.35 | 0.41 | |
3.2. Follow-Up of Laboratory Tests on the Day of Admission
|
Levosimendan (n = 23) |
Control (n = 135) |
p-value | |
| Blood counts | |||
| WBC (K/μL) at 6 h, mean ± SD | 14.0 ± 6.8 | 13.9 ± 6.0 | 0.98 |
| WBC (K/μL) at 24 h, mean ± SD | 14.4 ± 7.8 | 15.5 ± 7.0 | 0.51 |
| Neutrophils (K/μL) at 6 h, mean ± SD | 7.6 ± 5.8 | 7.9 ± 5.0 | 0.78 |
| Neutrophils (K/μL) at 24 h, mean ± SD | 12.4 ± 7.4 | 13.2 ± 6.5 | 0.57 |
| Hemoglobin (%) at 6 h, mean ± SD | 13.2 ± 2.4 | 12.5 ± 2.6 | 0.05 |
| Hemoglobin (%) at 24 h, mean ± SD | 12.1 ± 1.9 | 11.0 ± 2.5 | 0.06 |
| Platelet (K/μL) at 6 h, mean ± SD | 182.5 ± 74.8 | 189.3 ± 140.8 | 0.82 |
| Platelet (K/μL) at 24 h, mean ± SD | 123.5 ± 53.3 | 134.6 ± 72.0 | 0.48 |
| Biochemical indices | |||
| Troponin-I (ng/mL) at 6 h, mean ± SD | 6.9 ± 5.7 | 4.3 ± 3.8 | 0.08 |
| Troponin-I (ng/mL) at 24 h, mean ± SD | 110.2 ± 100.3 | 80.1 ± 80.8 | 0.11 |
| AST (U/L) at 6 h, mean ± SD | 249.6 ± 198.8 | 305.4 ± 311.4 | 0.46 |
| AST (U/L) at 24 h, mean ± SD | 577.6 ± 287.7 | 812.6 ± 1872.8 | 0.58 |
| ALT (U/L) at 6 h, mean ± SD | 125.2 ± 105.4 | 150.8 ± 177.6 | 0.53 |
| ALT (U/L) at 24 h, mean ± SD | 232.6 ± 180.3 | 272.7 ± 566.0 | 0.74 |
| BUN (mg/dL) at 6 h, mean ± SD | 21.0 ± 7.7 | 24.8 ± 20.6 | 0.41 |
| BUN (mg/dL) at 24 h, mean ± SD | 26.2 ± 6.1 | 28.9 ± 17.6 | 0.47 |
| Creatinine (mg/dL) at 6 h, mean ± SD | 1.5 ± 0.4 | 2.0 ± 2.0 | 0.22 |
| Creatinine (mg/dL) at 24 h, mean ± SD | 1.7 ± 0.4 | 2.0 ± 1.5 | 0.29 |
| Lactate (mmol/L) at 6 h, mean ± SD | 14.0 ± 6.3 | 16.0 ± 6.8 | 0.21 |
| Lactate (mmol/L) at 24 h, mean ± SD | 5.1 ± 2.8 | 7.2 ± 6.2 | 0.12 |
3.3. Analyses of Sequential Organ Failure Assessment (SOFA), Length of Stay, Clinical Outcomes, and Cerebral Performance Category (CPC)
|
Levosimendan (n = 23) |
Control (n = 135) |
p-value | |
| SOFA on day 1,mean±SD | 14.1 ± 1.9 | 14.4 ± 2.5 | 0.64 |
| SOFA on day 3, mean ± SD | 13.4 ± 2.6 | 15.8 ± 5.2 | 0.03 |
| SOFA on day 5, mean ± SD | 12.7 ± 3.3 | 16.0 ± 6.2 | 0.01 |
| SOFA on day 7, mean ± SD | 11.6 ± 3.4 | 16.2 ± 7.0 | 0.003 |
| Hospital stay (days), mean ± SD | 38.0 ± 21.7 | 24.5 ± 26.0 | 0.02 |
| ECMO weaning failure, n (%) | 3 (13.0) | 71 (52.6) | < 0.001 |
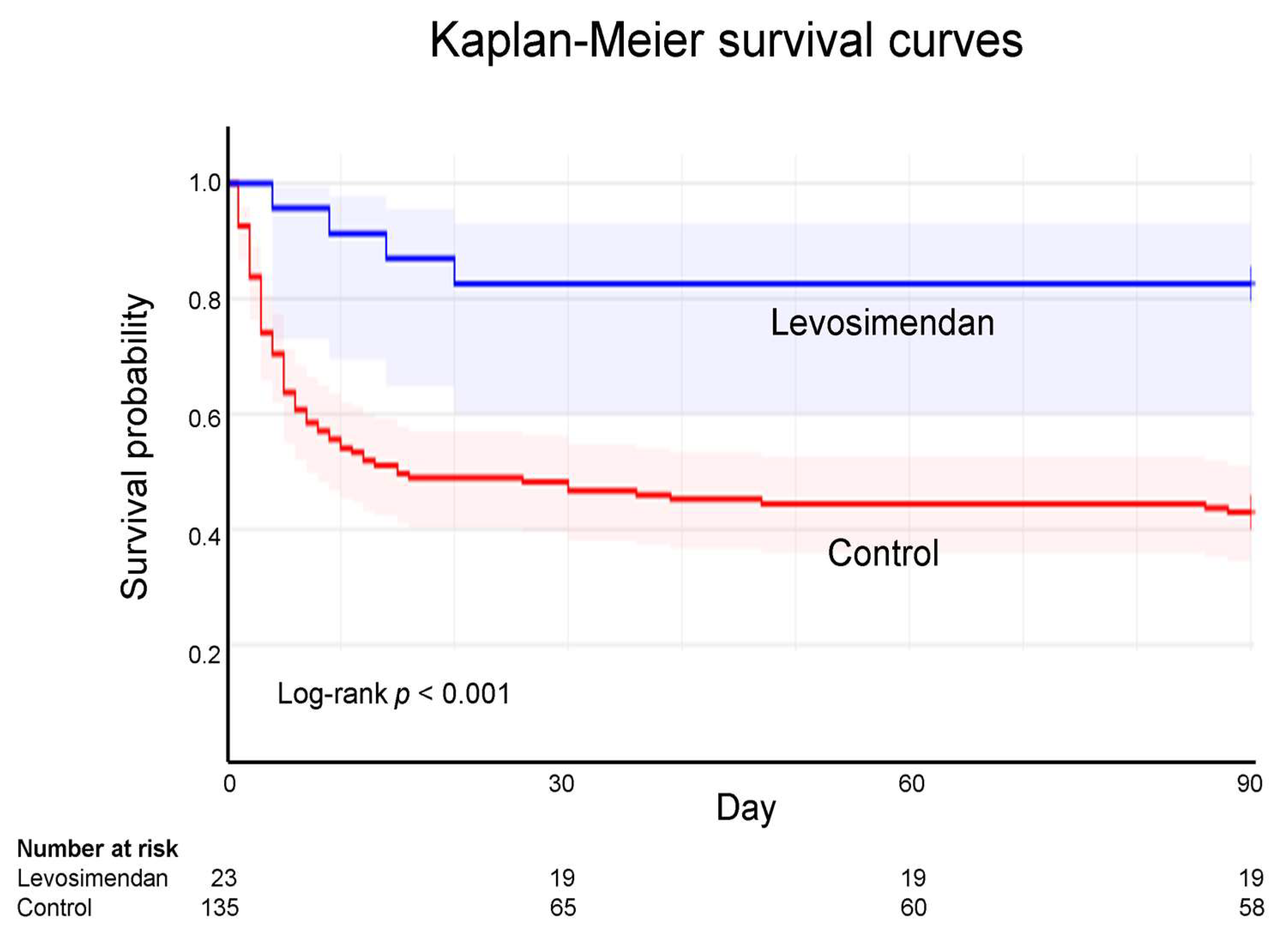
| 90-day mortality, n (%) | 4 (17.4) | 77 (57.0) | < 0.001 |
| 90-day poor neurological outcomes, n (%) | 11 (47.8) | 97 (71.8) | 0.06 |
| CPC sub-analyses at 90-day follow-up | |||
| CPC 1, n (%) | 6 (26.1) | 27 (20.0) | |
| CPC 2, n (%) | 6 (26.1) | 11 (8.1) | |
| CPC 3, n (%) | 5 (21.7) | 7 (5.2) | |
| CPC 4, n (%) | 2 (8.7) | 13 (9.6) | |
| CPC 5, n (%) | 4 (17.4) | 77 (57.0) | |
3.4. Kaplan-Meier Survival Curves of ECPR
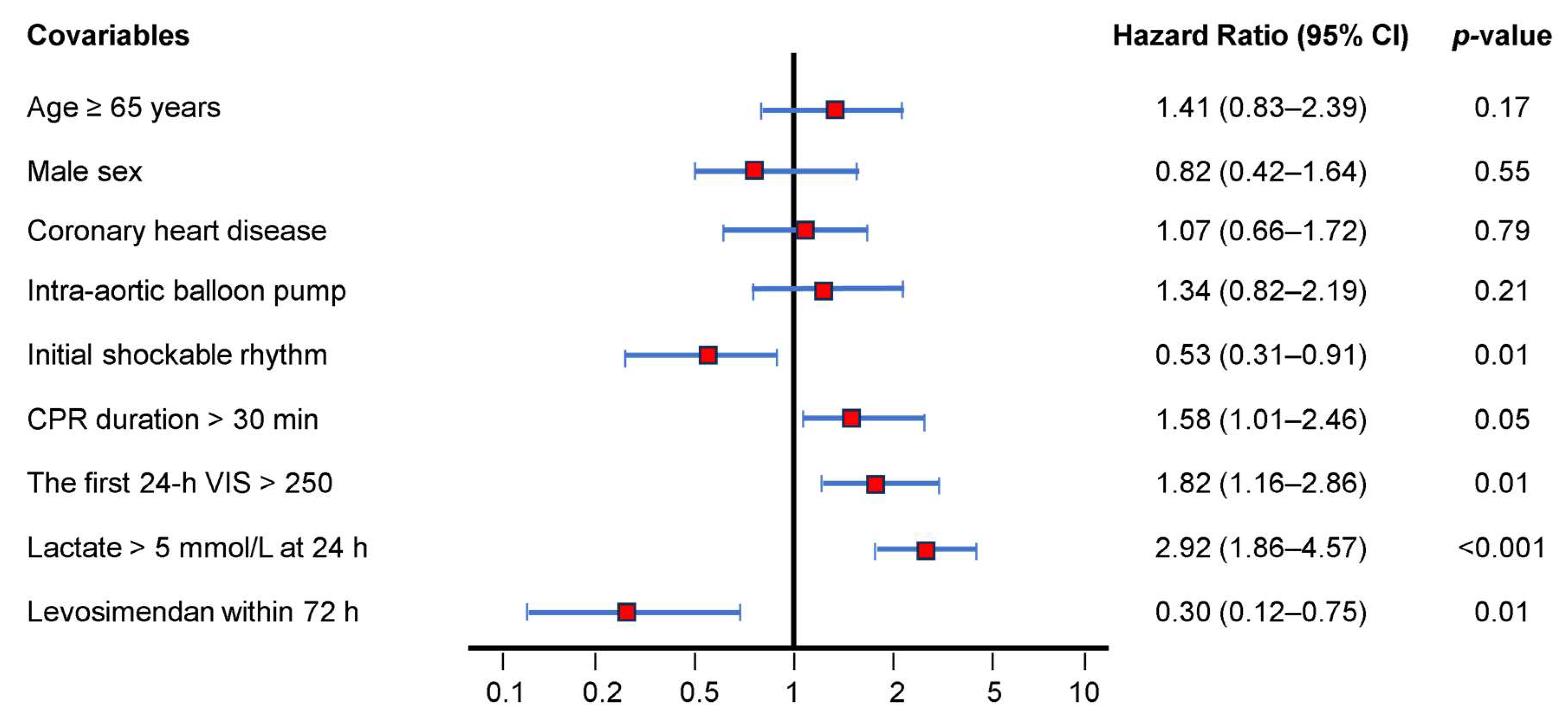
3.5. Ninety-Day Mortality Rate-Adjusted Covariables Using Cox Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAHP | Cardiac arrest hospital prognosis |
| CI | Confidence interval |
| CPC | Cerebral Performance Category |
| CPO | Cardiac power output |
| CPR | Cardiopulmonary resuscitation |
| ECPR | Extracorporeal cardiopulmonary resuscitation |
| ECMO | Extracorporeal membrane oxygenation |
| LVEF | Left ventricular ejection fraction |
| LVCI | Left ventricular cardiac index |
| OHCA | Out-of-hospital cardiac arrest |
| SOFA | Sequential Organ Failure Assessment |
| VIS | Vasoactive-inotropic score |
References
- Zheng J, Lv C, Zheng W, Zhang G, Tan H, Ma Y, et al. Incidence, process of care, and outcomes of out-of-hospital cardiac arrest in China: a prospective study of the BASIC-OHCA registry. Lancet Public Health. 2023;8:e923–32. [CrossRef]
- Kondo T, Araki T, Imaizumi T, Sumita Y, Nakai M, Tanaka A, et al. Prognosis in patients with cardiogenic shock who received temporary mechanical circulatory support. JACC Asia. 2022;3:122-34. [CrossRef]
- Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24:61. [CrossRef]
- Kim MW, Kim TH, Song KJ, Shin SD, Kim CH, Lee EJ, et al. Comparison between dispatcher-assisted bystander CPR and self-led bystander CPR in out-of-hospital cardiac arrest (OHCA). Resuscitation. 2021;158:64-70. [CrossRef]
- Narducci ML, Pedicino D. A new defibrillation strategy for refractory ventricular fibrillation during out-of-hospital cardiac arrest: are two better than one? Eur Heart J. 2023;44:919-20. [CrossRef]
- Mandigers L, Boersma E, den Uil CA, Gommers D, Bělohlávek J, Belliato M, et al. Systematic review and meta-analysis comparing low-flow duration of extracorporeal and conventional cardiopulmonary resuscitation. Interact Cardiovasc Thorac Surg. 2022;35:ivac219. [CrossRef]
- Miraglia D, Almanzar C, Rivera E, Alonso W. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest: a scoping review. J Am Coll Emerg Physicians Open. 2021;2:e12380. [CrossRef]
- Inoue A, Hifumi T, Sakamoto T, Kuroda Y. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients. J Am Heart Assoc. 2020;9:e015291. [CrossRef]
- Low CJW, Ramanathan K, Ling RR, Ho MJC, Chen Y, Lorusso R, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with cardiac arrest: a comparative meta-analysis and trial sequential analysis. Lancet Respir Med. 2023;11:883-93. [CrossRef]
- Low CJW, Ling RR, Ramanathan K, Chen Y, Rochwerg B, Kitamura T, et al. Extracorporeal cardiopulmonary resuscitation versus conventional CPR in cardiac arrest: an updated meta-analysis and trial sequential analysis. Crit Care. 2024;28:57. [CrossRef]
- Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV, et al. Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest. N Engl J Med. 2023;388:299-309. [CrossRef]
- Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396:1807-16. [CrossRef]
- Heringlake M, Alvarez J, Bettex D, Bouchez S, Fruhwald S, Girardis M, et al. An update on levosimendan in acute cardiac care: applications and recommendations for optimal efficacy and safety. Expert Rev Cardiovasc Ther. 2021 ;19:325-35. [CrossRef]
- Girardis M, Bettex D, Bojan M, Demponeras C, Fruhwald S, Gál J, et al. Levosimendan in intensive care and emergency medicine: literature update and expert recommendations for optimal efficacy and safety. J Anesth Analg Crit Care. 2022 ;2:4. [CrossRef]
- Luo JC, Zheng WH, Meng C, Zhou H, Xu Y, Tu GW, et al. Levosimendan to facilitate weaning from cardiorespiratory support in critically ill patients: A meta-analysis. Front Med (Lausanne). 2021;8:741108. [CrossRef]
- Tabi M, Burstein BJ, Ahmed A, Dezfulian C, Kashani KB, Jentzer JC. Shock severity and hospital mortality in out of hospital cardiac arrest patients treated with targeted temperature management. Shock. 2021;55:48-54. [CrossRef]
- Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-inotropic score: Evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. 2021;35:3067-77. [CrossRef]
- Na SJ, Chung CR, Cho YH, Jeon K, Suh GY, Ahn JH, et al. Vasoactive inotropic score as a predictor of mortality in adult patients with cardiogenic shock: Medical therapy versus ECMO. Rev Esp Cardiol (Engl Ed). 2019;72:40-7. [CrossRef]
- Pashkovetsky E, Gupta CA, Aronow WS. Use of levosimendan in acute and advanced heart failure: short review on available real-world data. Ther Clin Risk Manag. 2019;15:765-72. [CrossRef]
- Papp Z, Agostoni P, Alvarez J, Bettex D, Bouchez S, Brito D, et al. Levosimendan efficacy and safety: 20 Years of SIMDAX in clinical use. J Cardiovasc Pharmacol. 2020;76:4-22. [CrossRef]
- Desai HN, Sangurima L, Malik MM, Ganatra N, Siby R, Kumar S, et al. Therapeutic development of Levosimendan in acute and advanced heart failure: A systematic review. Cureus. 2023;15:e37844. [CrossRef]
- Glinka L, Mayzner-Zawadzka E, Onichimowski D, Jalali R, Glinka M. Levosimendan in the modern treatment of patients with acute heart failure of various aetiologies. Arch Med Sci. 2019;17:296-303. [CrossRef]
- Susilo H, Aldian FM, Wungu CDK, Alsagaff MY, Sutanto H, Multazam CECZ. Levosimendan, a promising pharmacotherapy in cardiogenic shock: A comprehensive review. Eur Cardiol. 2024;19:e21. [CrossRef]
- Brahmbhatt DH, Daly AL, Luk AC, Fan E, Billia F. Liberation form venoarterial extracorporeal membrane oxygenation: A review. Circ Heart Fail. 2021;14:e007679. [CrossRef]
- Lüsebrink E, Stremmel C, Stark K, Joskowiak D, Czermak T, Born F, et al. Update on weaning from veno-arterial extracorporeal membrane oxygenation. J Clin Med. 2020;9:992. [CrossRef]
- Quintero-Altare A, Flórez-Navas C, Robayo-Amortegui H, Rojas-Arrieta M, Tuta-Quintero E, Bastidas-Goyes A, et al. Boosting the beat: A critical showdown of Levosimendan and Milrinone in surgical and non-surgical scenarios: A narrative review. J Cardiovasc Pharmacol Ther. 2024;29:10742484241276431. [CrossRef]
- Luo JC, Zheng WH, Meng C, Zhou H, Xu Y, Tu GW, et al. Levosimendan to facilitate weaning from cardiorespiratory support in critically ill patients: A meta-analysis. Front Med (Lausanne). 2021;8:741108. [CrossRef]
- Bertini P, Paternoster G, Landoni G, Falcone M, Nocci M, Costanzo D, et al. Beneficial effects of levosimendan to wean patients from VA-ECMO: a systematic review and meta-analysis. Minerva Cardiol Angiol. 2023;71:564-74. [CrossRef]
- Gaisendrees C, Schlachtenberger G, Gerfer S, Krasivskyi I, Djordjevic I, Sabashnikov A, et al. The impact of levosimendan on survival and weaning from ECMO after extracorporeal cardiopulmonary resuscitation. Artif Organs. 2023 ;47:1351-60. [CrossRef]
- Ellouze O, Soudry Faure A, Radhouani M, Abou-Arab O, Besnier E, Moussa M, et al. Levosimendan in venoarterial ECMO weaning. Rational and design of a randomized double blind multicentre trial. ESC Heart Fail. 2021;8:3339-47. [CrossRef]
- Kaddoura R, Omar AS, Ibrahim MIM, Alkhulaifi A, Lorusso R, Elsherbini H, et al. The effectiveness of Levosimendan on veno-arterial extracorporeal membrane oxygenation management and outcome: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021;35:2483-95. [CrossRef]
- Liu Y, Zhang L, Yao Y, Li Y, Qin W, Li Y, et al. Effects of levosimendan on the outcome of veno-arterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. Clin Res Cardiol. 2024;113:509-21. [CrossRef]
- Sanfilippo F, Knight JB, Scolletta S, Santonocito C, Pastore F, Lorini FL, et al. Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Crit Care. ;21:252. [CrossRef]
- Hau M, Fong KM, Au SY. Levosimendan's effect on venoarterial extracorporeal membrane oxygenation weaning. Int J Artif Organs. 2022;45:571-9. [CrossRef]
- Mastoris I, Tonna JE, Hu J, Sauer AJ, Haglund NA, Rycus P, et al. Use of extracorporeal membrane oxygenation as bridge to replacement therapies in cardiogenic shock: Insights from the extracorporeal life support organization. Circ Heart Fail. 2022;15:e008777. [CrossRef]
- Lannemyr L, Ricksten SE, Rundqvist B, Andersson B, Bartfay SE, Ljungman C, wt al. Differential effects of Levosimendan and Dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: A randomized double-blind controlled trial. J Am Heart Assoc. 2018;7:e008455. [CrossRef]
- Tholén M, Ricksten SE, Lannemyr L. Effects of levosimendan on renal blood flow and glomerular filtration in patients with acute kidney injury after cardiac surgery: a double blind, randomized placebo-controlled study. Crit Care. 2021;25:207. [CrossRef]
- Kaddoura R, Orabi B, Omar AS, Ibrahim MIM, Alyafei SA, Alkhulaifi A, et al. The Role of Levosimendan in extracorporeal membrane oxygenation for refractory cardiac arrest. J Cardiothorac Vasc Anesth. 2025 :S1053-0770(25)00030-8. [CrossRef]
- Chen YW, Lee WC, Wu PJ, Fang HY, Fang YN, Chen HC, et al. Early Levosimendan administration improved weaning success rate in extracorporeal membrane oxygenation in patients with cardiogenic shock. Front Cardiovasc Med. 2022;9:912321. [CrossRef]
- Guilherme E, Jacquet-Lagrèze M, Pozzi M, Achana F, Armoiry X, Fellahi JL. Can levosimendan reduce ECMO weaning failure in cardiogenic shock?: a cohort study with propensity score analysis. Crit Care. 2020 ;24:442. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





