1. Introduction
In the recent decades, the world has witnessed an increasing emergence of antimicrobial resistance (AMR) primarily due to misuse and overuse of antimicrobials in both humans and animals [
1]. AMR is being considered as a silent pandemic. With many countries in the world facing serious public health crises due to Antimicrobial Resistance (AMR), it is crucial to explore the root causes of the problem [
2]. This exploration is necessary for implementing preventive measures both nationally and globally. Currently, there is limited knowledge about the actual causes of AMR, especially in middle and low-income countries. Basic information, supported by reliable data, regarding the use and misuse of antibiotics in human and veterinary medicine, as well as in plants, is either limited or absent. Without such information, it is challenging to determine the causes of AMR [
1].
Pakistan, with its available scientific resources, funding opportunities, and a deep interest in the subject, can be considered a model for other similar countries in investigating the causes of AMR.
Pakistan has a large rural and agriculture-based industry, animal husbandry plays an important role in the economy of Pakistan and is a major source of livelihood for many farmers. Livestock plays a significant role in socio-economic development of the country. In the year 2022-23 livestock sector contributed 14.3% of GDP to the national economy. The livestock sector provides employment to 30 million people especially of rural areas [
3]. The increasing demand for livestock due to urbanization, increase in population and export opportunities leads to the increasing trend of intensive farming in Pakistan. Therefore, farmers are extensively using antibiotics in food producing animals as growth promoters and for treatment and prophylaxis purposes. A recent study carried out in Pakistan to assess the knowledge, attitudes and practices (KAP) of field veterinarians regarding AMR and antimicrobial use (AMU) indicated irrational prescription of antibiotics including for treatment of viral diseases and prophylactic purposes [
4]. Most studies have shown increasing rates of resistance in both human and veterinary medicine, with multi-drug resistant organisms (MDROs) being isolated, with increasing frequency, across the country. The use of antimicrobial agents in animals, poultry and agriculture has recognized benefits but overuse has potentially serious implications for human health. Antimicrobial Stewardship Programs (ASP), Infection Control Programs and AMU/AMR monitoring and surveillance are the most effective immediate options for combatting and monitoring the trend of AMU and subsequently AMR. As such understanding the access to antimicrobials and mapping the supply chain is inevitable to identify areas of interventions for an effective ASP, in addition to other actions around AMU monitoring at farm-level and AMR surveillance in animals. Unfortunately, in Pakistan, the supply chain of antimicrobials has not been mapped yet, which limits country’s ability to develop and institutionalize a robust ASP.
Pakistan has initiated national AMR surveillance strategy for healthy and diseased food animals (Cattle, buffalo and poultry), two National Reference laboratories i.e., National Reference Laboratory for Poultry Diseases (NRLPD) and National Veterinary Laboratory (NVL) and nine peripheral laboratories [
5]. Due to inefficient Antimicrobial Stewardship Programs (ASP) and extensive use of antibiotics in Pakistan, it is important to monitor the quantities and usage patterns of antimicrobials in animals by analyzing the whole AMU supply chain in terms of registration, procurement, production, distribution, management and monitoring of usage of antimicrobials in Pakistan. The Ministry of National Food Security and Research (MoNFS&R) has been trying to share reliable annual data on antimicrobial usage in Pakistan to the World Organization for Animal Health (WOAH). However, there are still challenges faced in collection of quality and comprehensive data from various sources including the livestock and aquaculture industries, government authorities and other private sectors [
6]. The information on antimicrobial use (AMU) in animals is crucial for identifying potential risk factors and enabling effective control and prevention of antimicrobial resistance (AMR) in animals. This requires a comprehensive surveillance system to monitor the use and fate of antimicrobials in animal diseases in Pakistan. However, before implementing such a surveillance system, it is essential to gather basic information and understand the steps in the flow of antimicrobials, from manufacturing to administration, as well as their presence in animal-origin food items. A functional national monitoring system for antimicrobials used in animals can provide valuable information about the type and quantity of antimicrobials used and this information can be correlated with the resistance patterns to provide policy decisions.
The MoNFS&R, owing to interest toward tackling AMR in food animals and with the support of WOAH and Fleming Fund, organized a workshop in March 2020 on monitoring of AMU in the animal health sector in Pakistan. The key objectives were to map the antimicrobial supply chain and support establishment of a system for monitoring the quantities and usage patterns of antimicrobial agents used in animals in Pakistan. The aim of this paper is to present a study conducted to map and understand the antimicrobial supply chain in livestock and poultry sector through inputs from stakeholders gathered for this purpose. This information will assist in designing and initiating a monitoring and surveillance system for AMU among various livestock sectors in Pakistan.
1.1. Methodological Approach
Chief Veterinary Office/ Livestock Wing of the Ministry of National Food Security and Research, being a lead authority for the animal health sector, organized the AMU monitoring workshop with technical and financial assistance from the WOAH Regional Representation Office in Japan and the Fleming Fund Country Grant, Pakistan.
1.2. Selection Criteria for Stakeholders:
To ensure diverse representation of participants from both public and private sectors for the AMU monitoring workshop, following selection criteria were considered:
- (a)
Institutional representation: Stakeholders or key institutions engage in import, regulation, distribution, and use of antimicrobials, such as regulatory bodies, human health sector, veterinary departments, Customs, veterinary educational establishments, fisheries and aquaculture, drug quality testing laboratory, and research institutes.
- (b)
Expertise and Knowledge: Participants with expertise in veterinary medicine, animal husbandry, pharmaceuticals, public health, and regulatory affairs related to antimicrobials.
- (c)
Geographical Representation: Participation from all regions or provinces within the country to capture regional variations in AMU practices and regulations.
Based on the set criteria, international, federal and provincial authorities, organizations, and stakeholders, as given in
Table 1, were identified and invited to the workshop.
1.3. Development of Activity Sheets
Activity sheets were developed prior to the workshop to gather information on the AMU supply chain and monitoring, structured according to the specifications provided in
Table 3,
Table 4 and
Table 5 and
Figure 1.
1.4. Stakeholder Consultation
-
(a)
Mapping of AMU Supply Chain: During the first day of the workshop, regulatory authorities and other participants were divided into groups to map the sources of the supply chain of antimicrobial agents intended for use in animals. However, before seeking this important information, the objectives of the activity were discussed. To sensitize the participants and emphasize the significance of national data reporting and regulatory procedures at federal and provincial levels, detailed presentations were delivered. These covered topics such as the global AMU database and the importance of national data reporting (presented by OIE/WOAH), the regulatory process around antimicrobial import, registration, sale, and usage (presented by the Drug Regulatory Authority of Pakistan), the regulatory frameworks for monitoring antimicrobial usage (presented by the Livestock & Dairy Development Department Punjab), and the import process and real-time surveillance capabilities for antimicrobials (presented by the Customs Department). This preparation aimed to ensure effective group discussions focused on mapping the AMU supply chain.
-
(b)
AMU Monitoring: On second day of the workshop, following the technical presentations on status and activities on AMR and AMU by various stakeholders, the participants were divided into three groups based on their expertise to brainstorm on(i) current legislation and governance status for monitoring of AMU in Pakistan to identify the key government regulators and their roles in regulating AMU along the supply chain; (ii) national AMU data collection and monitoring system to identify types of AMU data that each stakeholder can provide to the MoNFS&R, and (iii) monitoring of AMU at end-user level to identify priority activities to enhance national AMU monitoring system in Pakistan. The aim of this exercise was to consider the various steps and conditions for marketing and using various antimonial products among livestock species in Pakistan. Prior to the workshop, the organizers drafted a map of antimicrobial chain supplies and shared it with the breakout groups to confirm and modify the pathways for AMU in livestock species.
Result compilation and concurrence: At the end of each exercise, every group presented their findings and based on that, the drafted results were developed with the consensus of the participants.
2. Results
2.1. The AMU Supply Chain of Pakistan
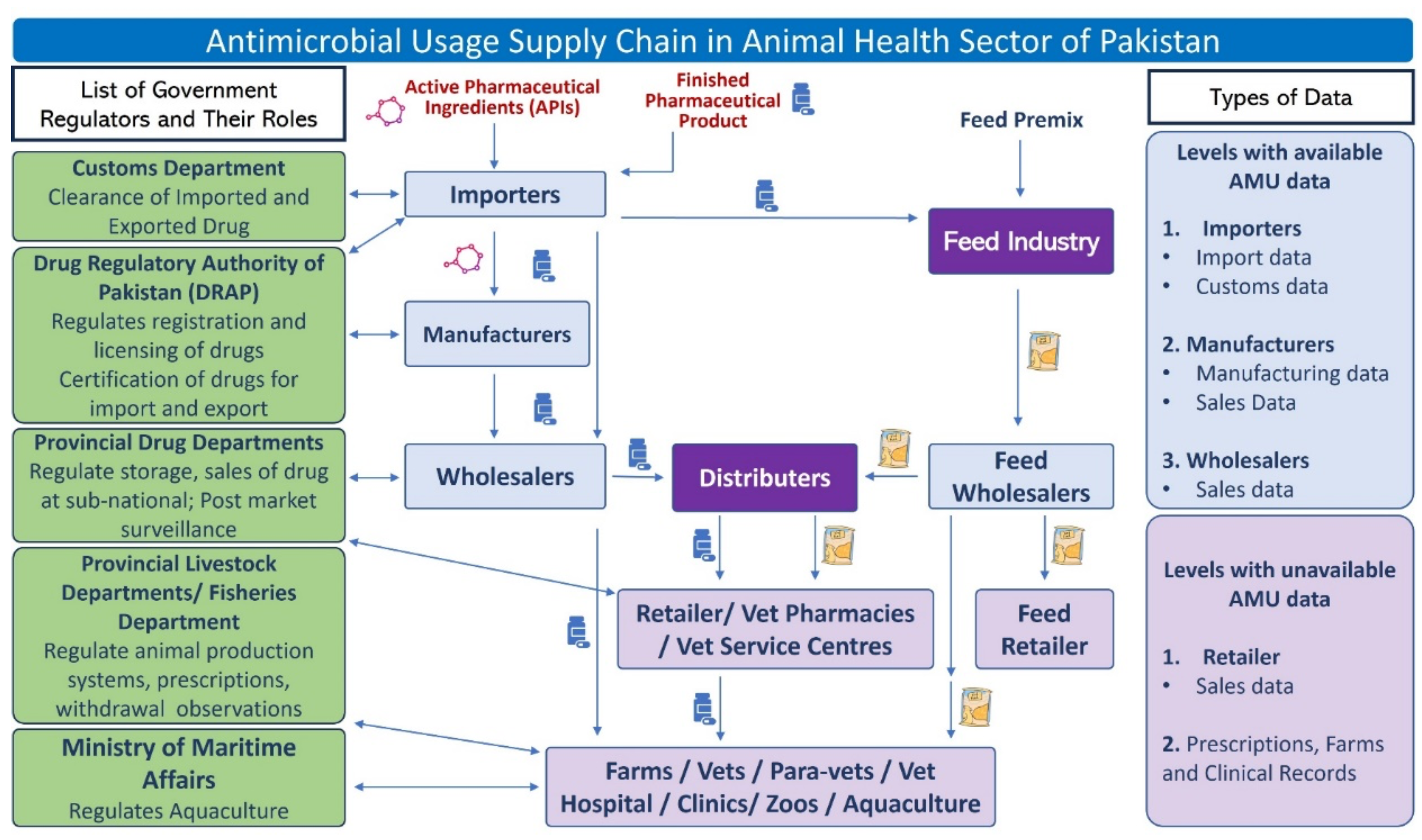
Based on the findings and/or inputs of all groups, a flow diagram (
Figure 1) on antimicrobial supply chain in Pakistan was developed with the consensus of the participants. Presently, the drug regulatory authority of Pakistan (DRAP) of the federal government regulates the import, manufacture, registration, and pricing of antimicrobials while provincial governments regulate distribution, sale and post-marketing inspection (jointly with DRAP) in the provinces. The Pakistan Customs department deals with the clearance of import and export of drugs. Pakistan Customs have strong real-time surveillance system and therefore the MoNFS&R can access the data bank of the Customs department for data on antimicrobials and active pharmaceutical ingredients (APIs) imported into Pakistan. The provincial livestock departments regulate animal production systems, prescriptions, withdrawal observations in livestock farms and clinics.
Only pharmaceutical companies are allowed to import APIs whereas finished pharmaceutical products (FPPs) can be imported by pharmaceutical companies and importers. As per the AMU supply chain, the importers import FPPs and sell to wholesalers, retailers, feed industry and sometimes even to farms directly. Feed industries import feed premix containing antibiotics and purchase FPPs from importers to be added to animal feed based on demand. Medicated feed (containing antibiotics) and FPPs are then distributed to retailers, veterinary pharmacies, and veterinary service centres through distributors. Antimicrobials are ultimately used by farms, veterinarians, para-veterinary staff, veterinary hospitals/clinic, zoos and aquaculture. As for AMU data, presently, only higher-level AMU data are available such as from importers (import data), customs office (customs data), manufacturers (manufacturing at higher-level) and wholesalers (sales data). Currently, no AMU data at the lower level of the AMU supply chain is available such as retailer/pharmacy sale data, farm level usage data, prescription data from clinics and veterinary hospitals etc.
Currently, at the federal level, there are no regulations on import, manufacture, registration, and sale of animal feed premix (medicated feed) containing antimicrobials. However, some provinces such as Punjab have legislations regulating use of medicated poultry feed and have also list of permissible and non-permissible antimicrobials used in poultry feed. According to the Punjab Poultry Production Act-2016 [
7], antimicrobials such as colistin and ciprofloxacin are banned for use as antimicrobial growth promoter in poultry feed. The government wants to take cautious approach in banning the use of Antimicrobial Growth Promotors (AGPs) in poultry production and will develop step wise approach in phasing out the use of antimicrobials for growth promotion.
2.2. Legislation and Governance status for Monitoring of AMU in Pakistan
There is currently no policy or legislation system to monitor the use of antimicrobials at the national and provincial level (
Table 3). There is a need to review and update the existing DRAP Act by adding relevant clauses to accommodate the requirement for regulations of veterinary medicines including antimicrobials. This should be led by DRAP and Ministry of Health MoNHSRC with the collaboration of MoNFS&R. Likewise the revised national legislations should cover reporting of antimicrobial usage at prescriber and farm level and should also include monitoring of premixes containing antimicrobials by the provincial livestock departments in collaboration with MoNFS&R and DRAP. Regulations of unauthorized usage of antimicrobials need to be developed by reviewing the existing legislations by Provincial Health Care Commissions and Pakistan Veterinary Medical Council involving Provincial Livestock Departments including developing a legal framework for prescription requirement for the veterinary practitioners. The legislations related to acceptable daily intake and maximum residue levels are already available but need to be implemented through Provincial Livestock Departments involving DRAP and MoNFS&R.
2.3. National AMU Data Collection and Monitoring System
Table 4 shows the types of AMU data that each stakeholder can provide to the Ministry of National Food Security and Research. Currently Pakistan does not have a national AMU data collection and monitoring system for antimicrobials used in animals. Therefore, it is important to develop a national AMU monitoring system to be led by the Animal Husbandry Commissioners office (AHC) of MoNFS&R jointly with the Fisheries Development Commissioner (FDC), Ministry of Maritime (MoMT) in collaboration with Drug Regulatory Authority of Pakistan, Pakistan Customs Department, and Provincial Livestock Departments. It is important to make a list of critical antimicrobials that are of importance for Pakistan based on WHO list of critical antimicrobials and WOAH list of veterinary critical antimicrobials.
The AHC office and FDC should also make a list of importers of antimicrobials with the support of DRAP and Pakistan Customs Department that could be contacted for submission of import data. The AHC and FDC office should also develop necessary guidelines for monitoring of antimicrobials used in animals and share with enforcement agencies such as DRAP, Pakistan Customs, Provincial Livestock Departments, provincial drug regulation authorities and health departments. The national AMU monitoring system should clearly spell out the roles and responsibilities of relevant stakeholders identified in the AMU supply chain particularly for their roles in collection and sharing of AMU data with the MoNFS&R. A national AMU database system should be developed that will also be the repository for AMU data from animal sector. It is important to advocate awareness on the importance of AMU monitoring so that AMU data collection can be harmonised at all levels of the government and the private sector.
2.4. Monitoring of AMU at End-User Level
AMU Monitoring: According to identified priority activities to enhance national AMU monitoring system (
Table 5), Pakistan lack an established system for monitoring of AMU. To address this, a comprehensive surveillance plan and legislation were proposed, involving collaboration between federal and provincial entities, as well as both public and private sectors.
Guidelines/SOP’s: Similarly, no guidelines and standard operating procedures (SOPs) for AMU monitoring exist. The forward strategy should involve collaborative efforts from federal and provincial authorities with academia, livestock departments, research institutes, associations, and fish departments.
Development monitoring system: Currently, there is a lack of a centralized database for AMU monitoring. The proposed way forward includes initiating a pilot study in various production systems, which require collaboration between federal and provincial bodies, as well as involving livestock departments and associations.
Publications: Due to limited published data, proposed plans include conducting more studies in collaboration with academia, research institutes, livestock departments, and associations.
Implementations Challenges: Several challenges, such as a lack of awareness, trained manpower, funding, and political interest, were recognized. To address these challenges, budget allocation, political will, and training were identified as impactful solutions. This comprehensive approach requires collaboration between federal and provincial entities and planning and finance departments.
Roles and responsibilities: Clarification and establishment of roles and responsibilities are essential, focusing on federal legislation. This requires legislation, implementation, data analysis, and reporting.
Knowledge Attitude Practices surveys: In 2020, there was no planned activity for KAP (Knowledge, Attitudes, and Practices) surveys. The suggested plans include a comprehensive study with a multi-sectorial approach, raising awareness, and observing AMR awareness week. These activities should be led by livestock departments, with the collaboration of academia, research institutes, NGOs, associations, and farmers.
3. Discussion
Pakistan imports an abundant quantity of antimicrobials for human and animal sectors. The value of imports of antibiotics as commodity to Pakistan was
$127 million in 2020, which indicated 5.8% increase compared to 2019 [
8]. Monitoring animal antimicrobial usage is crucial to curb irrational use. Currently, DRAP oversees federal regulation of antimicrobial import, manufacture, registration, and pricing for animals, while provinces, jointly with DRAP, regulate distribution, sale, and post-marketing inspection. Pakistan lacks a national monitoring system for animal antimicrobial usage. WOAH recommends implementing such a system to prevent misuse and ensure access to quality veterinary medicines [
9]. Therefore, regulatory authorities can use findings of current study to make informed decisions regarding the regulation, import, and manufacture of antimicrobials for animals. By identifying gaps in existing legislation and governance related to AMU, this study suggests a roadmap for regulatory improvements. Similarly, suggested national AMU data collection and monitoring system can help track and manage antimicrobial use, preventing misuse and overuse.
Overall, Pakistan’s veterinary drugs supply chain is fragmented. At national level it is regulated by the DRAP Pakistan which does not have in-house veterinary specific expertise and coordinate with the CVO office for veterinary specific consultations. This is a major gap which results in certain regulatory discrepancies e.g., the veterinary feed additives are not regulated by the DRAP however significant amount of antibiotics are being imported in country under the label of growth promoters. This also leads to inconsistent data of the veterinary antimicrobial imports and usage in country. Certain other factors also impede the collection of quantitative data on AMU, including ‘lack of regulatory framework’, ‘lack of coordination between national authorities and private sector’, ‘lack of tools and human resources’ and ‘insufficient regulatory enforcement’ [
10]. The countries in Europe have led from the front in effective AMU monitoring in animal sector that have resulted in reduction of AMR over the years. For instance, Denmark has developed the Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP), using one health approach, which not only collect antimicrobial consumption (AMC)/AMU data from both human and animal sectors but also monitor the AMR. This program works through the collaboration of Ministry of Health, the Ministry of Higher Education and Science, and the Ministry of Environment and Food [
11]. Denmark is also a part of European Surveillance of Veterinary Antimicrobial Consumption (ESVAC), which had been launched in 2009 for the harmonization of antimicrobial usage data in some European countries. For this purpose, they have developed the method for standardizing antimicrobial sales and designed the format to collect data from all member countries [
12]. This approach can be implemented at national level in countries like Pakistan involving all provinces/states. A drug distribution framework has been decided and strictly followed in the USA [
13], which makes it easier to collect data on AMC and monitor the antimicrobials in the country. In parallel, the USA has Animal Health Institute as a trade association of manufacturers of pharmaceuticals and animal health care products, that releases AMU/AMC estimate every year. This association also reports the total active ingredient manufactured and used in growth promotors products. The US-Food and Drug Authority (FDA), in collaboration with animal health industry, has also updated the guidelines for industry to make changes in antibiotic labels to ensure judicious use of medically important antibiotics.
A functional national monitoring system for AMU in animals can provide valuable information about the type and quantity of antimicrobials used and this information can be correlated with the resistance patterns to make policy decisions. There is a need to develop integrated national AMU database to integrate data collected from all the private sectors/companies/importers/distributors and end users dealing with antimicrobials. Based on the outputs of this workshop and the AMU data available with the end users in Pakistan, a potential flow sheet for Point Prevalence Survey (PPS) for the estimation of AMU was designed. Following this, PPS surveys have been conducted in commercial broiler [
14] and dairy [
15] production of Pakistan. These surveys provide high-resolution data on farm-level use of antimicrobials including seasonal and geographic differences in AMU. The findings of these studies clearly indicate the need to institute a robust national AMU monitoring system in Pakistan involving the key stakeholders at all levels. Based on the findings of activity on monitoring of AMU at end user level, Knowledge, Attitude and Practices (KAP) surveys on AMU for field veterinarians and farmers were designed and conducted at national level, covering all geographical areas of Pakistan. Another highly significant consequence of this activity was the collection of antimicrobial imported data from Drug and Regulatory Authority of Pakistan, which was subsequently provided to World Organization for Animal Health (WOAH) as per described format by WOAH candidates. Sharing insights and data on AMU in the livestock sector enhances Pakistan's participation in global initiatives addressing AMR. It fosters collaboration with international organizations, promoting a unified response to a shared public health threat. Therefore, activities under this study are highly important as a direction for other countries. The study also highlights the need for improved legislations and enhanced enforcement of regulations to ensure prudent use of antimicrobials in the livestock sector.
Way Forward
Considering the availability of financial resources and data, developing a national level AMU data collection system from sales and import data according to the WOAH methodology can be considered as a starting point for Pakistan while in parallel exploring details of AMU situation by using PPS. The development of a national AMU monitoring system in Pakistan will help understand the current situation of national monitoring of AMU in Pakistan in livestock and poultry sectors, including legislation and governance system, trends in the import of antimicrobials, status of AMU data monitoring (including AMU data collection, analysis, publication and sharing within and outside Pakistan, and roles and responsibilities of stakeholders in AMU data sharing. This includes other sectors such as customs, agriculture, importers and distributors/retailers (medical pharmacies, feed agents), and private farms including those importing informally.
Author Contributions
Conceptualization: M.A., R.W.U., K.D. and J.W; Methodology: M.F.T., R.W.U., J.A. M.S., and M.U.Z; Investigation: R.W.U., K.D., J.W., M.F.T and M.U.Z; Data curation: R.W.U., S.U.K.B., M.F.T., M.U.Z and U.T; Writing – Original Draft Preparation: S.U.K.B., M.F.T., M.U.Z., H.I., R.W.U. and U.T; Writing – Review & Editing: M.F.T., M.U.Z., M.A., H.I., K.D., M.S., J.A. and J.W.; Supervision: M.A., R.W.U., M.F.T. and M.U.Z; Project administration: M.A; Funding acquisition: M.F.T., M.U.Z. and M.A. .
Funding
This study was supported by the Fleming Fund Country Grant for Pakistan, grant number FF16-206. The Fleming Fund is a UK aid investment programme to tackle antimicrobial resistance in low- and middle-income countries around the world and is managed by the UK Department of Health and Social Care. The WOAH Regional Representation for Asia and the Pacific also provided funds to conduct the national AMU monitoring workshop. .
Conflicts of Interest
Authors declared no conflict of interest.
References
- Khan, M.S.; Durrance-Bagale, A.; Legido-Quigley, H.; Mateus, A.; Hasan, R.; Spencer, J.; Hanefeld, J. 'LMICs as Reservoirs of AMR': A Comparative Analysis of Policy Discourse on Antimicrobial Resistance with Reference to Pakistan. Health Policy Plan 2019, 34(3), 178–187. [Google Scholar] [CrossRef]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Sci. OA 2021, 7, FSO736. [Google Scholar] [CrossRef] [PubMed]
- Government of Pakistan (2023). Economic Survey of Pakistan. Finance Division, Economic Advisor’s Wing, Islamabad.
- Saman, A.; Chaudhry, M.; Ijaz, M.; Shaukat, W.; Zaheer, M.U.; Mateus, A.; Rehman, A. Assessment of Knowledge, Perception, Practices and Drivers of Antimicrobial Resistance and Antimicrobial Usage Among Veterinarians in Pakistan. Prev. Vet. Med. 2023, 212, 105836. [Google Scholar] [CrossRef] [PubMed]
- Animal Husbandry Commissioner. (2021). National Surveillance Strategy for Antimicrobial Resistance in Healthy Food Animals. Animal Husbandry Commissioner, Livestock Wing, Ministry of National Food Security and Research, Government of Pakistan, Islamabad, Pakistan.
- Qiu, Y.; Ferreira, J.P.; Ullah, R.W.; Flanagan, P.; Zaheer, M.U.; Tahir, M.F.; Alam, J.; Hoet, A.E.; Song, J.; Akram, M. Assessment of the Implementation of Pakistan’s National Action Plan on Antimicrobial Resistance in the Agriculture and Food Sectors. Antibiotics 2024, 13(3), 206. [Google Scholar] [CrossRef] [PubMed]
- Government of the Punjab. (2017). The Punjab Gazette. Law and Parliamentary Affairs Department, Lahore Pakistan. https://poultry.punjab.gov.pk/system/files/PPPRULE2017.pdf.
- Annual International Trade Statistics by Country (HS02). (2021). Trend Economy. Accessed in July 2021. Retrieved from https://trendeconomy.com/data/h2/Pakistan/2941.
- Kotwani A., Joshi J. (2020). Why We Need Smart Regulation to Contain Antimicrobial Resistance. Down to Earth. https://www.downtoearth.org.in/blog/health/why-we-need-smart-regulation-to-contain-antimicrobial-resistance-74311 (Accessed in August 2021).
- World Organization for Animal Health (WOAH). (2017). WOAH annual report on antimicrobial agents intended for use in animals. 2nd Report. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/Annual_Report_AMR_2.pdf (Accessed on August 2021).
- Anonymous. (2016). DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Danish Integrated Antimicrobial Resistance Monitoring and Research Programme, Denmark, 2017. http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202013/DANMAP%202013.ashx (accessed July 2021).
- European Medicines Agency. (2011). Trends in the Sales of Veterinary Antimicrobial Agents in Nine European Countries (2005-2009) (EMA/238630/2011). https://www.ema.europa.eu/en/documents/report/trends-sales-veterinary-antimicrobial-agents-nine-european-countries_en.pdf (Access July 2021).
- Viola, C.; DeVincent, S.J. Overview of Issues Pertaining to the Manufacture, Distribution, and Use of Antimicrobials in Animals and Other Information Relevant to Animal Antimicrobial Use Data Collection in the United States. Preventive Veterinary Medicine 2016, 73(2-3), 111-131.
- Umair, M.; Tahir, M.F.; Ullah, R.W.; Ali, J.; Siddique, N.; Rasheed, A.; Akram, M.; Zaheer, M.U.; Mohsin, M. Quantification and Trends of Antimicrobial Use in Commercial Broiler Chicken Production in Pakistan. Antibiotics 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.U.; Mohsin, M.; Umair, M.; Abubakar, M.; Rasheed, A.; Ullah, R.W.; Tahir, M.F. Farm-level study on the trends and quantitates of antimicrobial use in commercial dairy in Pakistan. Annual Conference of Research Workers in Animal Diseases, Chicago, IL, United States, December 3-7, 2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).