Over the last 30 years since postural orthostatic tachycardia syndrome (POTS) was defined in scientific literature, numerous mechanistic factors have been identified as its possible pathophysiology, including the involvement of the central nervous system. POTS, one of the most common disorders of the autonomic nervous system, is now an accepted frequent sequala of SARS-CoV-2 infection, and autonomic dysfunction more broadly is one of the major components of Long COVID (1-3). Pre-pandemic, POTS was known to occur after a viral or bacterial infection in at least 40% of patients and was thought to be of heterogeneous and complex pathophysiology involving central and peripheral autonomic nervous system networks, small fiber neuropathy, cerebral hypoperfusion, mast cell hyperactivity and autoimmunity (4). In fact, given its multitude of CNS symptoms and a positive response to CNS-penetrating medications, including stimulants, in many patients, POTS has been considered as a possible CNS disorder (4), but studies on neuroimaging and CNS pathophysiology in POTS have been extremely limited. One study before COVID-19 pandemic, using (1H) magnetic resonance spectroscopy to quantify markers of neuronal and glial integrity, demonstrated evidence of neuroinflammation at the dorsal medulla in pediatric patients with orthostatic intolerance vs. healthy controls (5). In their study, dorsal medulla was hypothesized to be an important locus and area of interest in patients with orthostatic intolerance and postural tachycardia – a potential localization that made sense because the nuclei of the vagus nerve were located in this area (5).
With the renewed interest in POTS and autonomic dysfunction as a result of SARS-CoV-2 pandemic, we are now able to elucidate more precisely and confirm the pre-pandemic hypothesis that POTS is a central nervous system disorder affecting the dorsolateral inferior medulla (4). Recently, in an innovative and highly informative study utilizing ultra-high field (7T) quantitative susceptibility mapping of the brain in patients with post-Covid symptoms after hospitalization, Rua et al. identified increased MR susceptibility in the medulla, pons and midbrain areas of the brainstem, specifically in reticular formation and raphe areas of the inferior medulla (6). Their results suggest neuroinflammation in these regions though further studies of a large cohort are necessary to confirm these findings. While the study participants were not specifically tested for POTS, autonomic dysfunction is one of the major mechanisms of lingering symptoms after SARS-CoV-2, in both hospitalized patients and those with mild infection that necessitates its own diagnostic and therapeutic approaches (2,3). In the National Academies of Science definition of Long COVID, autonomic dysfunction, including POTS – one of the most common autonomic disorders – is listed as a major feature of Long COVID (3). Although the mechanisms of Long COVID have not been fully delineated, immunologic, autoimmune, endothelial, autoimmune and hypercoagulable alterations have been identified (3,7). We can, therefore, consider findings from studies on patients with Long COVID to be relevant to patients with POTS, or at least 40% of patients with POTS whose illness began after an infection.
Dorsolateral inferior medulla as a possible localization of Long COVID, post-COVID dysautonomia and POTS in general is a reasonable consideration from the standpoint of clinical-anatomical correlation (4-6). First, the reticular formation serves as a relay between peripheral and central nervous system in how brainstem controls cardiopulmonary functions, respiration and consciousness. The major functions of the reticular formation are modulation of arousal, consciousness, circadian rhythm, sleep-wake cycles, coordination of somatic motor movements, cardiovascular and respiratory control, pain modulation, and habituation (8). Cardiovascular control is modulated by the vasomotor center present in the medulla. The central areas, which play a role in the autonomic rhythms of respiration, are located caudally in the reticular formation near the junction of the pons and the medulla. These centers are also associated with the cranial nerve motor nuclei of the trigeminal, facial, glossopharyngeal, vagus, and hypoglossal nerves to coordinate the complex task of respiration (8)
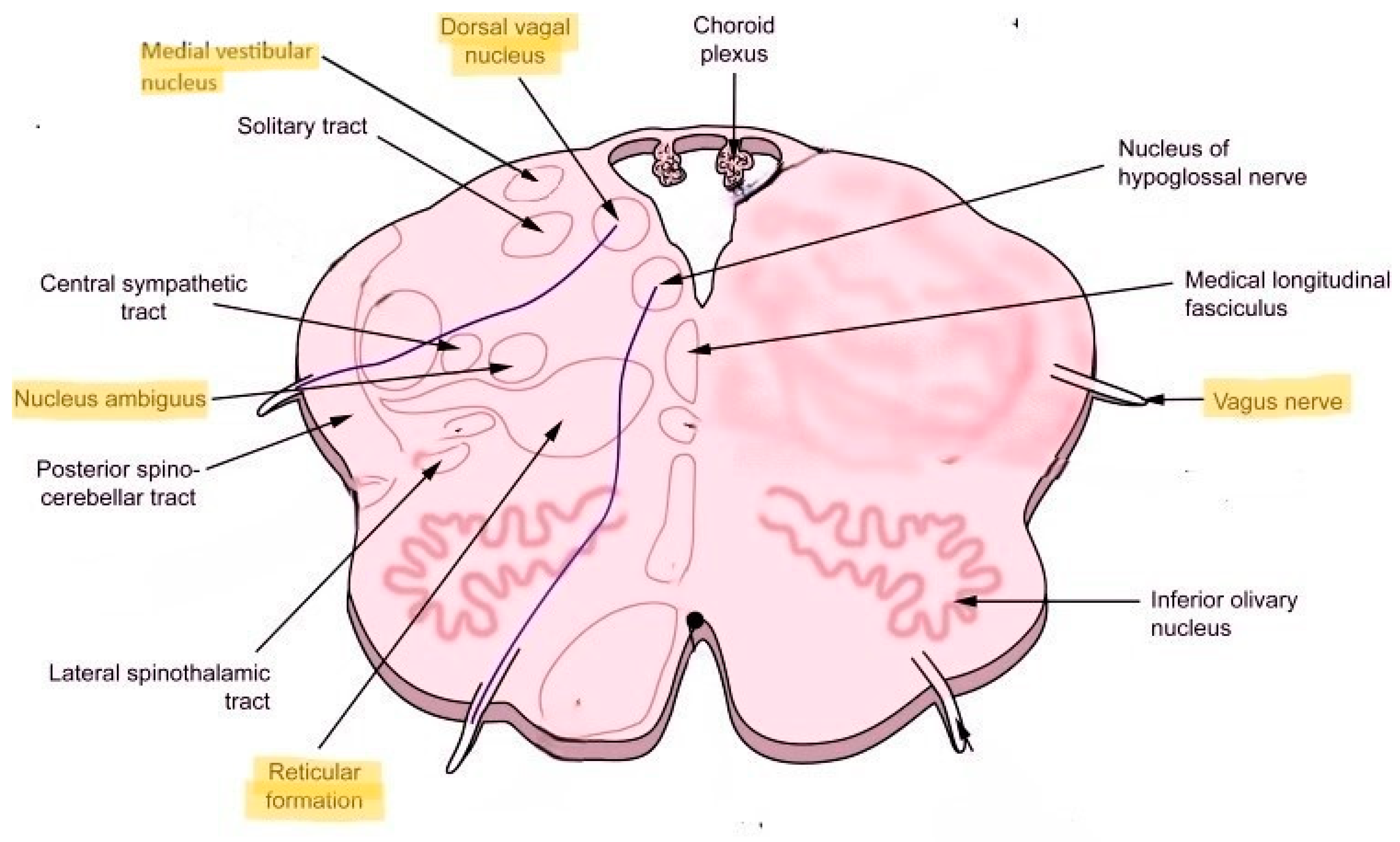
Second, nucleus ambiguus, which carries some of the preganglionic parasympathetic fibers, is located proximal to the reticular formation (
Figure 1). The vagus nerve and some of its fibers that come out of nucleus ambiguus carry the parasympathetic nerve fibers and innervate the heart and lungs. Disturbance of the parasympathetic outflow via the vagus nerve may result in reduced heart rate variability and respiration control underlying autonomic dysfunction. Located more posteriorly is the dorsal nucleus of the vagus nerve, which is a major carrier of the parasympathetic output to the heart and lungs. Located more laterally is the medial vestibular nucleus that’s important in the control of head, neck and body position as well as equilibrium and balance (
Figure 1). Since Long COVID commonly manifests with dizziness, fatigue, headache and blood pressure, heart rate and temperature dysregulation, localization to the dorsolateral medulla with possible neuroinflammation involving nucleus ambiguus, dorsal nucleus of the vagus and medial vestibular nucleus seems plausible from the standpoint of clinical-anatomical correlation.
Finally, Rua et al. specifically name nuclei raphe pallidus and obscurus as having increased susceptibility on 7T MRI in their study (6). These nuclei appear to be highly relevant to the central autonomic networks and brainstem-controlled immunologic response. Specifically, nucleus raphe pallidus is known to mediate the tachycardia response and appears to be involved in the activation of a fever as an immunoreaction (9). Tachycardia is a defining feature of POTS, and both tachycardia and recurrent fever are common manifestations of Long COVID and other post-acute infectious syndromes.
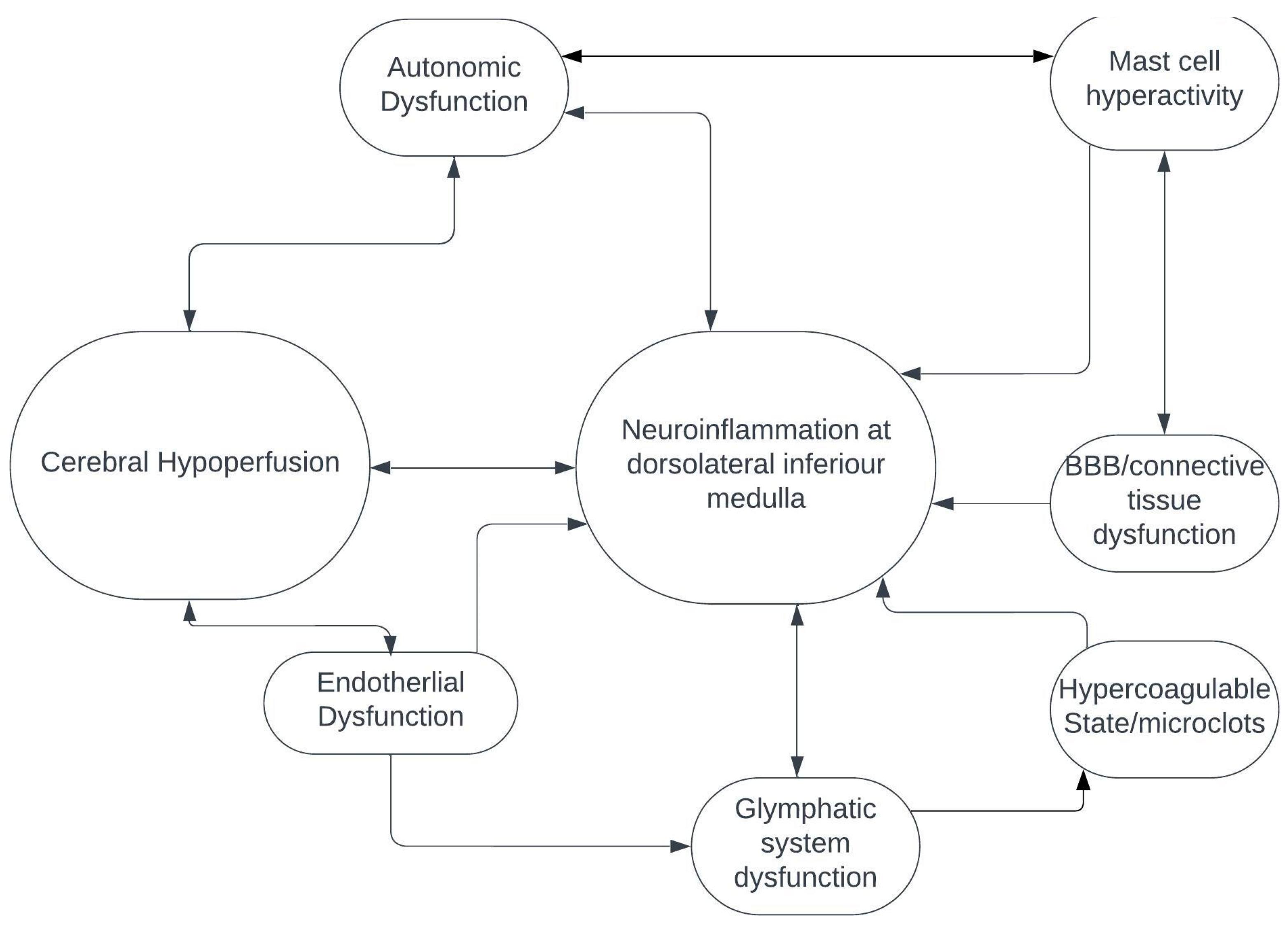
Cerebral hypoperfusion is one of the major mechanisms of POTS, autonomic dysfunction, myalgic encephalomyelitis/chronic fatigue syndrome and probably Long COVID (4). How cerebral – and, in particular, brainstem – hypoperfusion fits with neuroinflammation of the dorsolateral inferior medulla is currently unknown and needs to be investigated. It’s possible that small penetrating arteries coming off the vertebrobasilar arterial system and supplying the brainstem may be damaged from endothelial dysfunction, hypercoagulable state and possible microclots, all of which appear to be important in the pathogenesis of Long COVID (
Figure 2) (7). Additionally, and/or alternatively, alterations in the glymphatic system may result in inadequate venous drainage of dorsolateral medulla, venous congestion and toxin accumulation, further leading to neuroinflammation (
Figure 2) (10). Finally, abnormalities in the connective tissue itself, possibly resulting from neuroinflammatory and immune-mediated pathways that include hyperactivated mast cells, could alter the composition of venous and arteriolar walls and the blood-brain barrier, which could, in turn, result in abnormal vascular contractility and stiffening, thereby causing decreased perfusion of the brainstem (
Figure 2) (7,10).
In summary, Rua et al. identified evidence of neuroinflammation using 7T MRI in previously hospitalized patients with post-Covid symptoms at specific regions of the brainstem that are critical to the autonomic nervous system control of the organs and vital physiologic processes, including respiration, heart rate, blood flow, digestive system, bladder function and probably immunologic response (6). The highly diverse and multisystemic symptoms displayed by most patients with Long COVID correlate with dysfunction and structural abnormalities seen on 7T MRI of the dorsolateral inferior medulla, which could also be the localization for POTS and autonomic dysfunction more broadly, unrelated to SARS-CoV-2 infection. Further studies involving advanced neuroimaging techniques and animal models with immunohistochemical brainstem tissue assessments are needed to understand how and why possible neuroinflammation at the dorsolateral inferior medulla may occur in patients with Long COVID, POTS and other disorders involving autonomic dysfunction and cerebral hypoperfusion. Already preliminary evidence suggests that neuroinflammation at the dorsolateral inferior medulla is a possible central nervous system localization for POTS and Long COVID (4-6).
Conflict of Interest
none
References
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res 2021; 69: 205-211. [CrossRef]
- Blitshteyn S, Whiteson JH, Abramoff B et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. 2022;14: 1270-1291. [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. 2024. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences. Washington, DC: The National Academies Press. [CrossRef]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J Neurol 2022; 269: 725-732. [CrossRef]
- Wagoner AL, Olson JD, Westwood BM, et al. Children with orthostatic intolerance exhibit elevated markers of inflammation in the dorsal medulla. Am J Physiol Heart Circ Physiol 2019; 317: H323–H329.
- Rua C, Raman B, Rodgers CT et al. Quantitative susceptibility mapping at 7 T in COVID-19: brainstem effects and outcome associations. Brain 2024; October 7. [CrossRef]
- Turner S, Khan MA, Putrino D, Woodcock A, Kell DB, Pretorius E. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab 2023; 34: 321-344. [CrossRef]
- Mangold SA, Das JM. Neuroanatomy, Reticular Formation. 2023 Jul 24. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024 Jan.
- Nakamura K, Matsumura K, Kaneko T et al. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 2002; 22: 4600-4610. [CrossRef]
- Wu L, Zhang Z, Liang X et al. Glymphatic system dysfunction in recovered patients with mild COVID-19: A DTI-ALPS study. iScience 2023; 27: 108647. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).