Submitted:
04 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. In Vivo Experimental Procedures and Ethics Statement
2.2. Biochemical Evaluations of Serum Hepatic Parameters, Lipid Profile, and Inflammatory/Immune Mediators
2.3. Mitochondrial Bioenergetics and Redox Status Evaluation
2.4. ROS Assay
2.5. RNA Extraction and Semi-Quantitative Real-Time (RT)-PCR
2.6. Statistical Analysis
3. Results
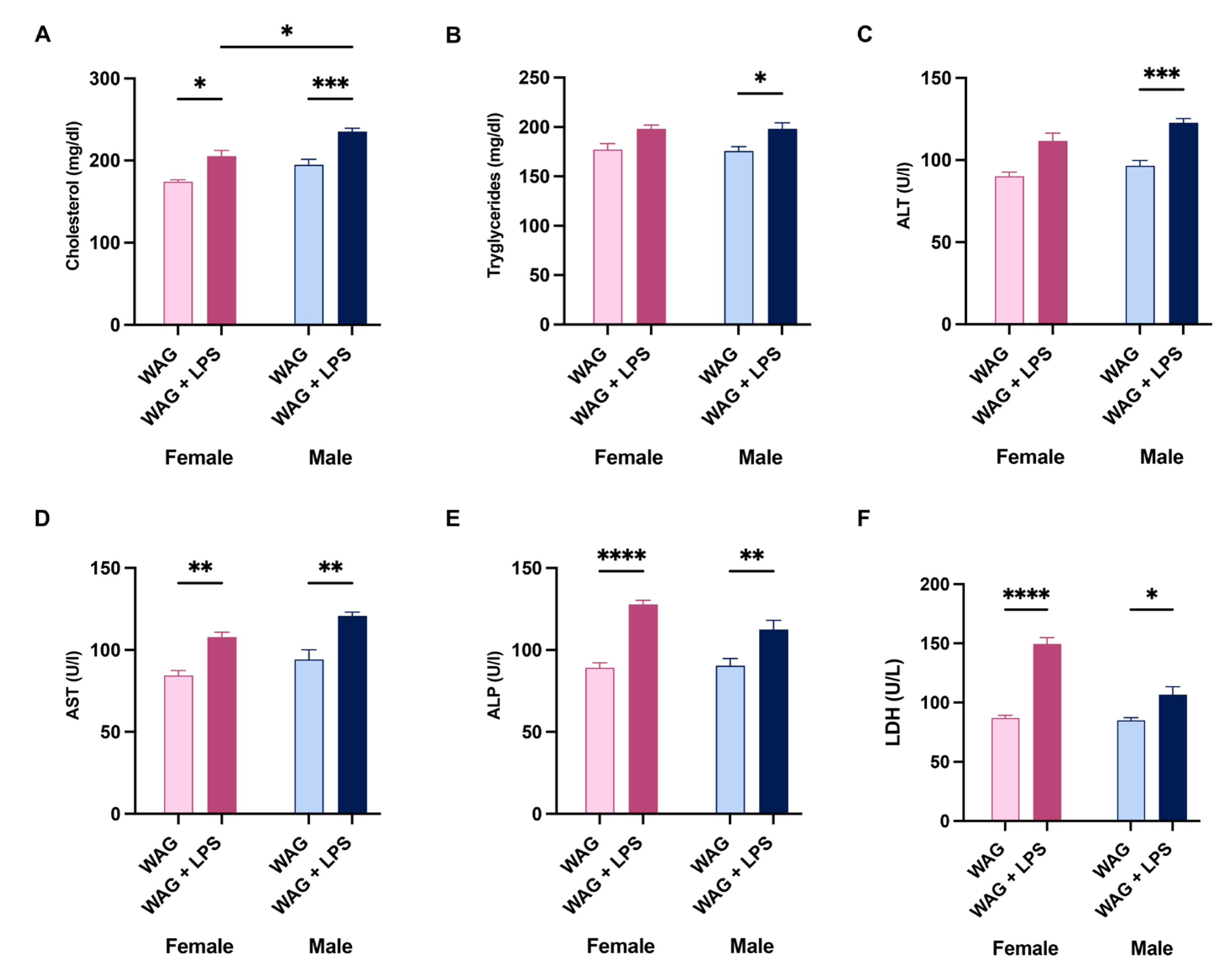
3.1. Gender Differences and LPS Effect on Biochemical Parameters and Inflammatory Mediators in Serum of WAG/Rij Rats
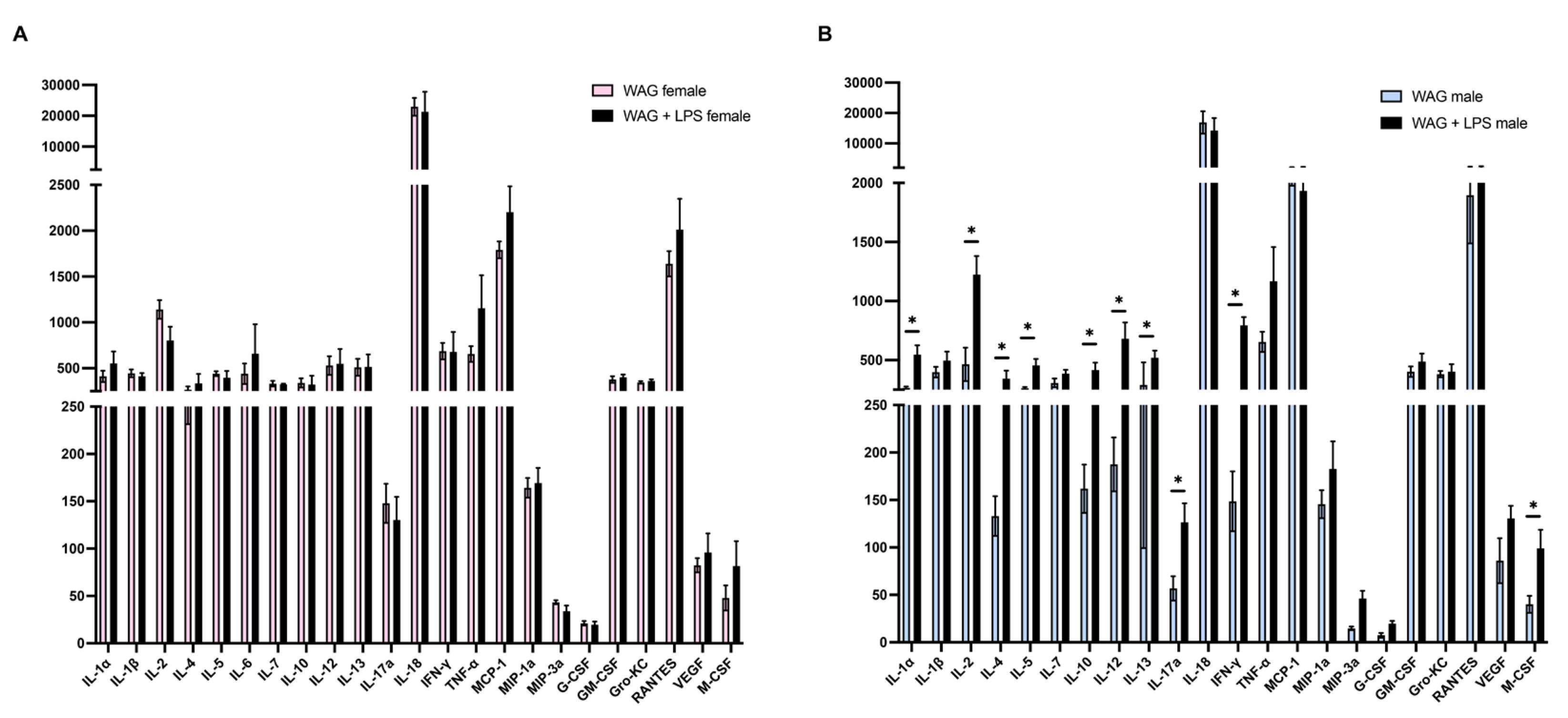
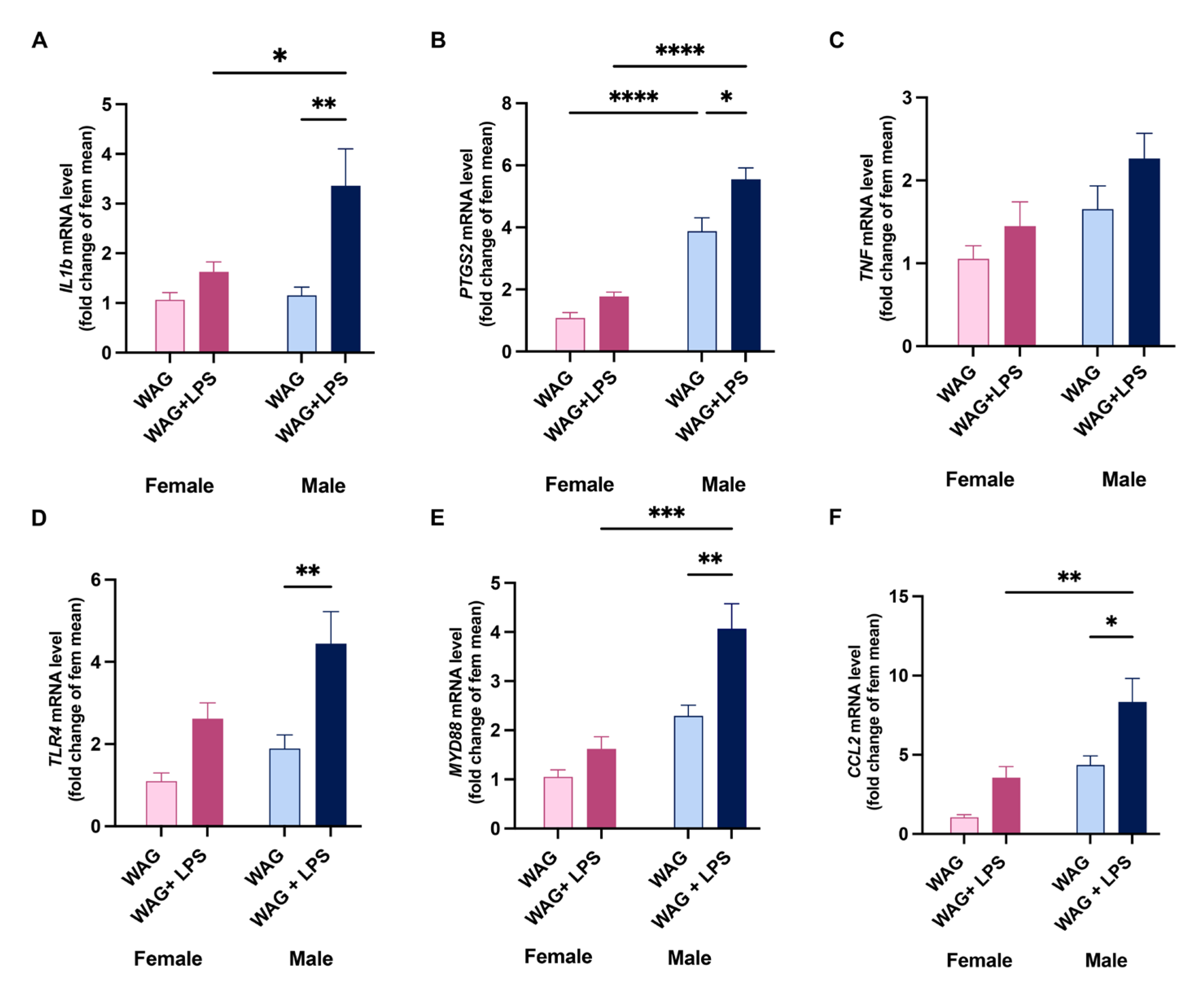
3.2. Hepatic Inflammation and Immune Response of Male and Female Epileptic Rats: Effect of an Early LPS Challenge
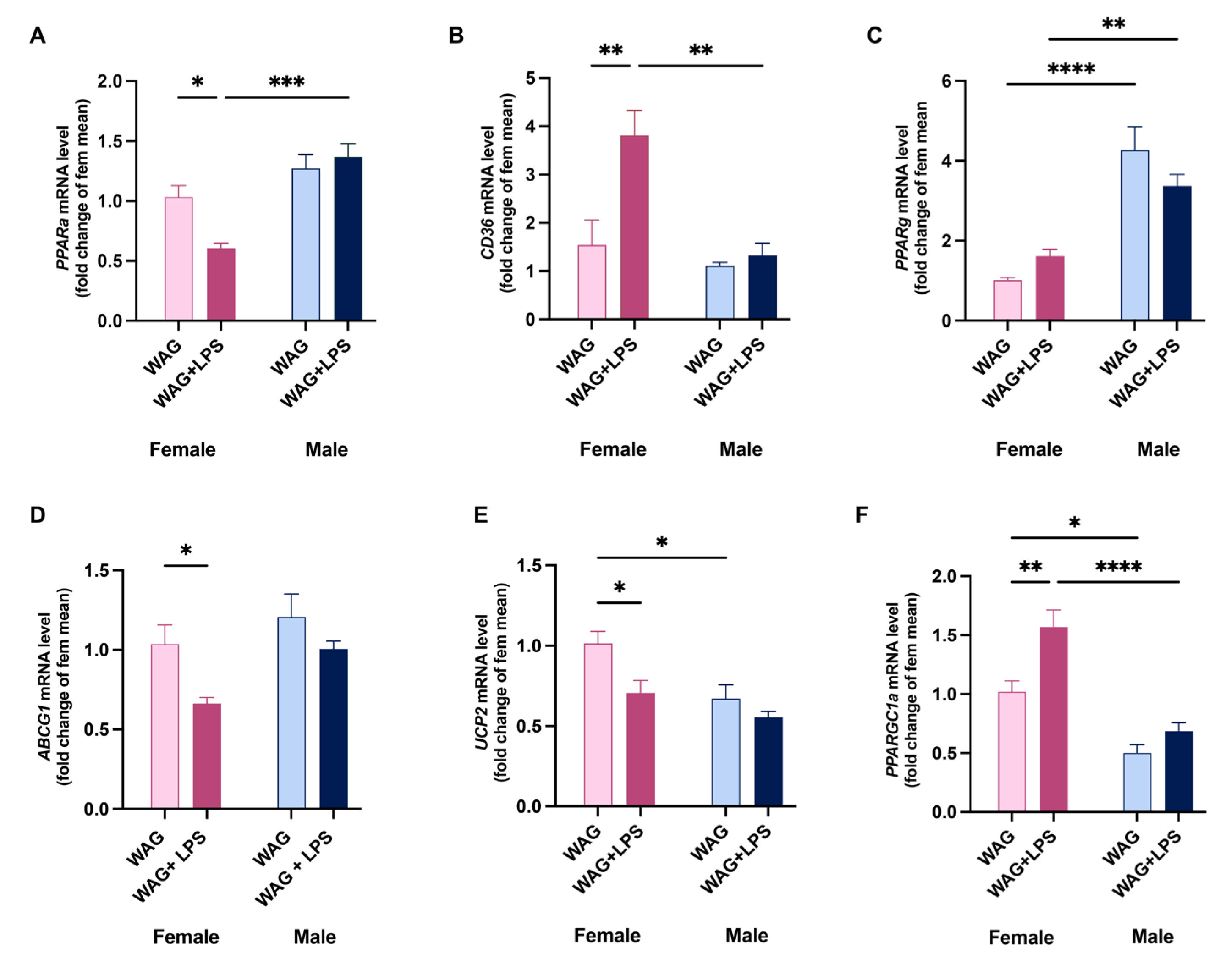
3.3. LPS-Driven Metabolic Alterations in Liver of Epileptic Rats: Sex-Related Differences
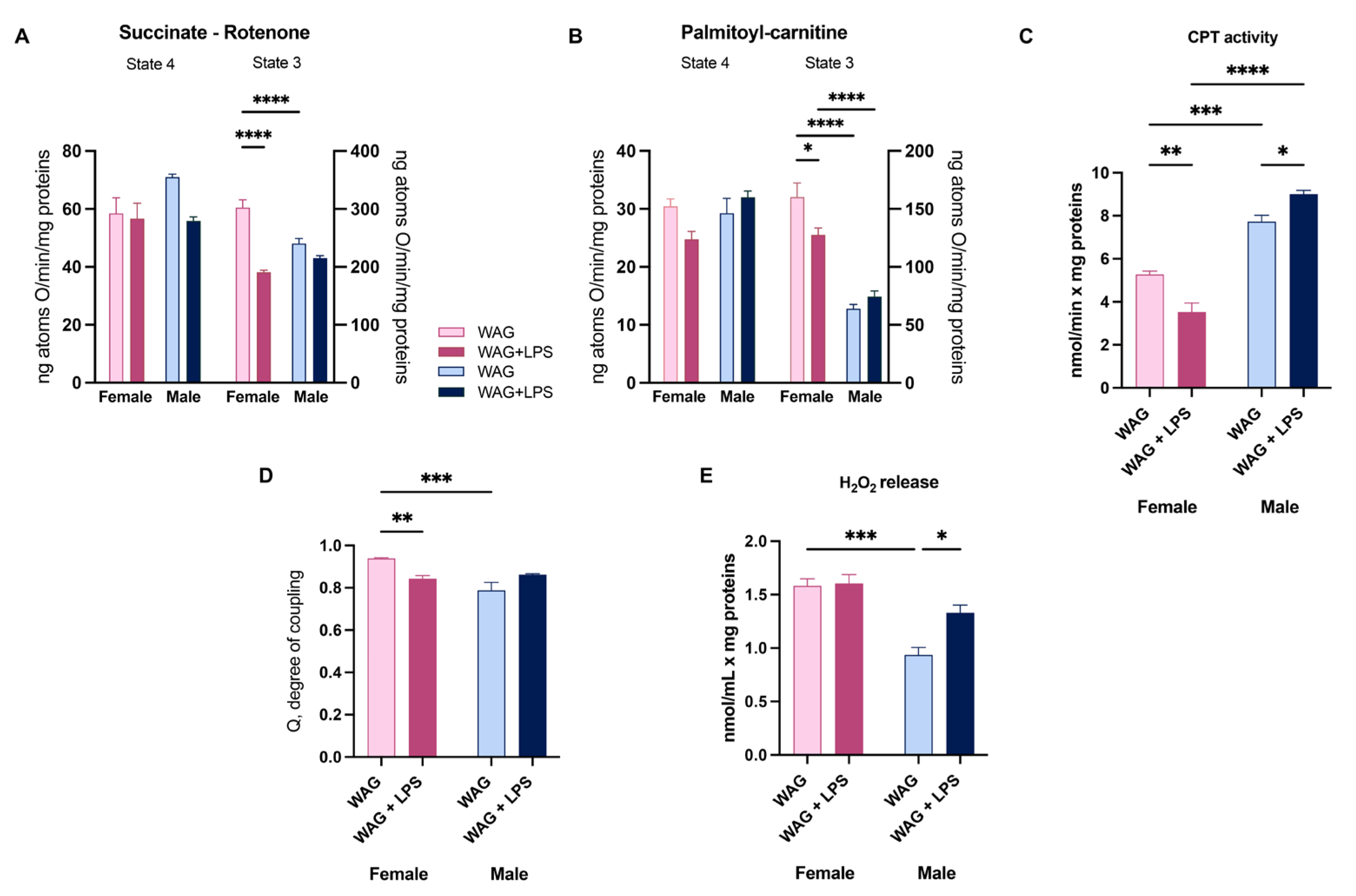
3.4. Effect of LPS on Hepatic Mitochondrial Bioenergetics in Male and Female Epileptic Rats
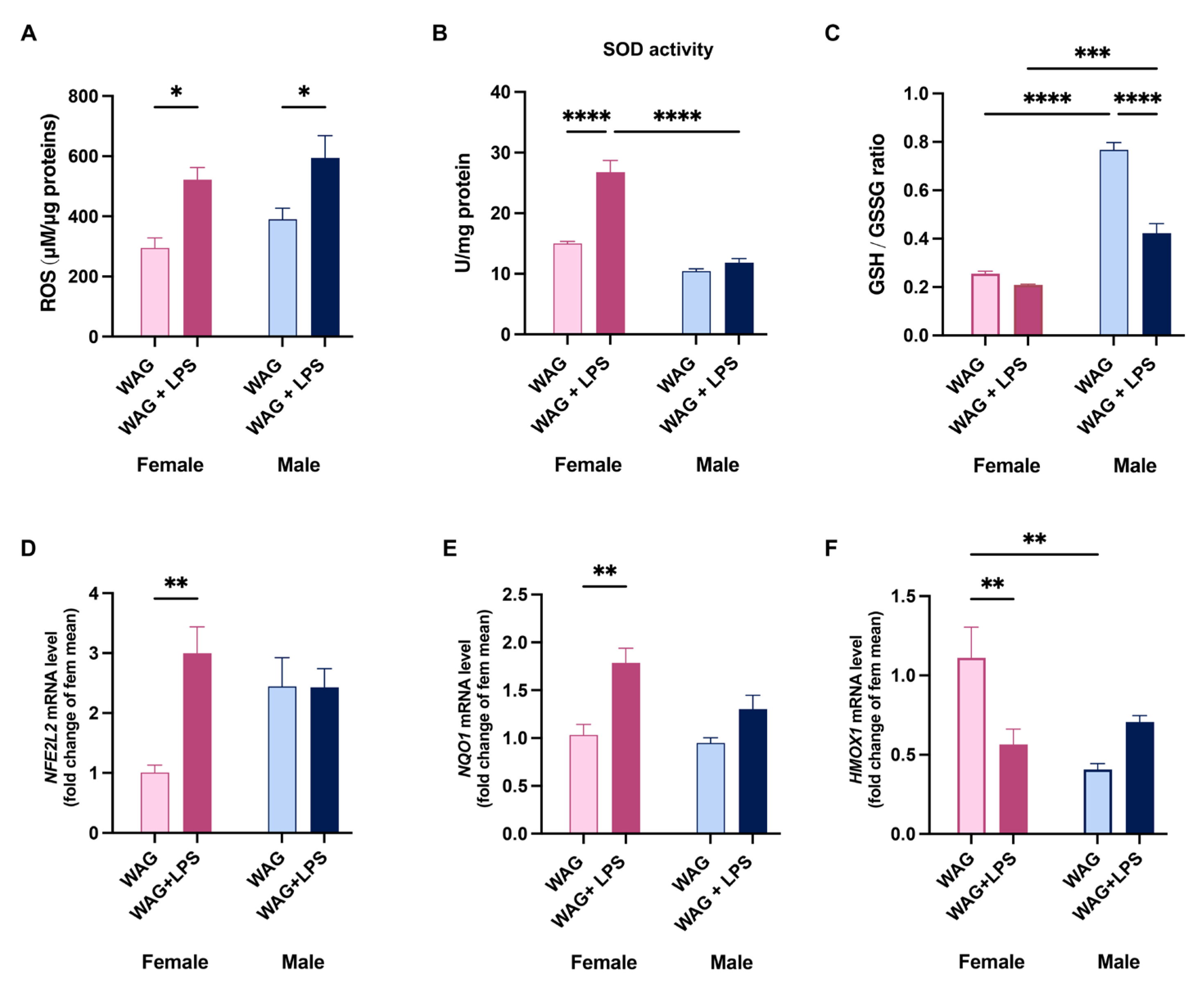
3.5. Sex-Related Susceptibility to Oxidative Stress of WAG/Rij Rats: Effect of LPS Injection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
1. Supplementary Methods
1.1. Biochemical Evaluations of Serum Inflammatory/Immune Mediators
1.2. Electroencephalogram (EEG) Recordings of 3-Month-Old Male WAG/Rij Rats
1.3. Statistical Analysis
2. Supplementary Results
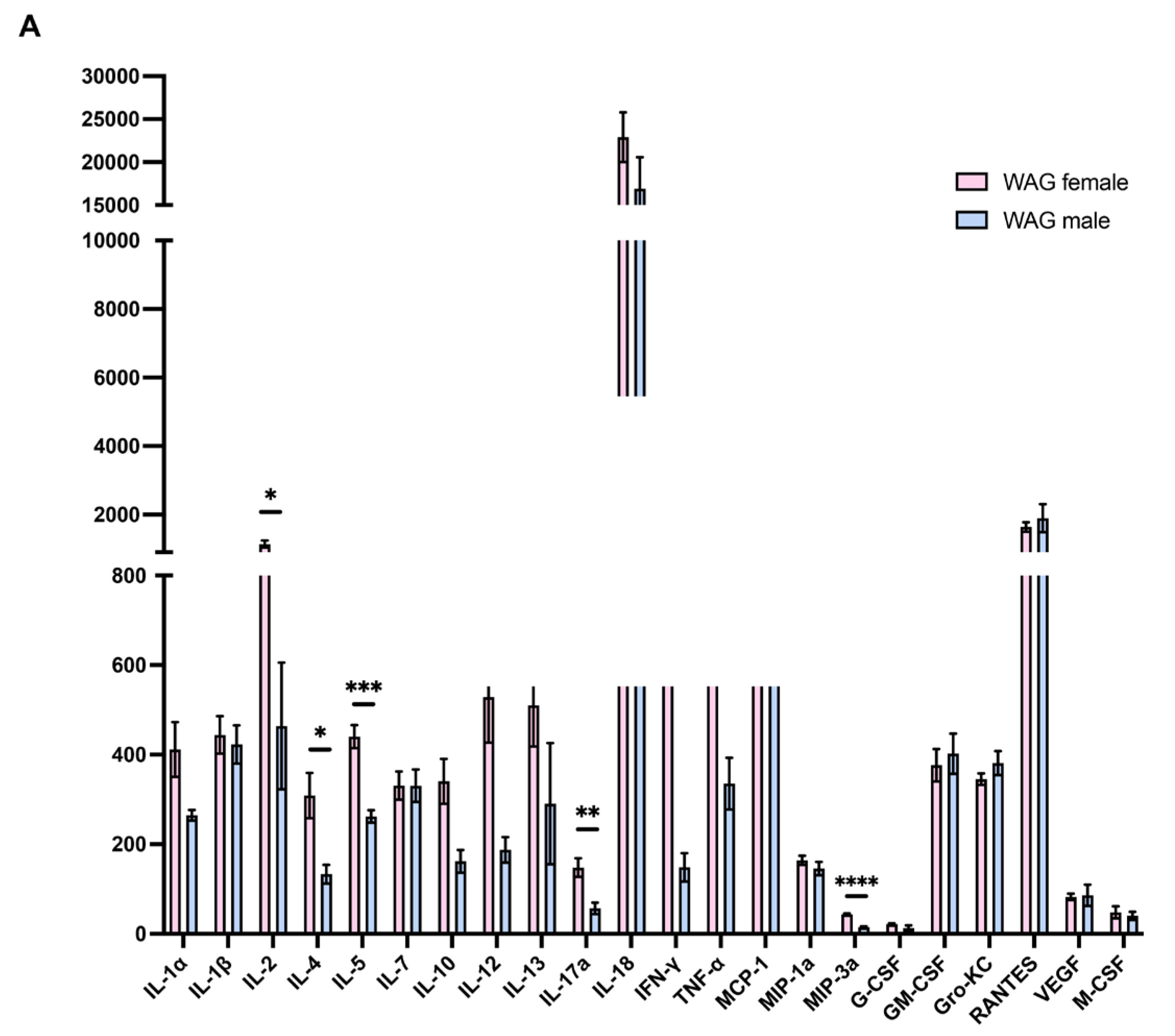
2.1. Intergender Differences on Inflammatory Mediators in Serum of WAG/Rij Rats
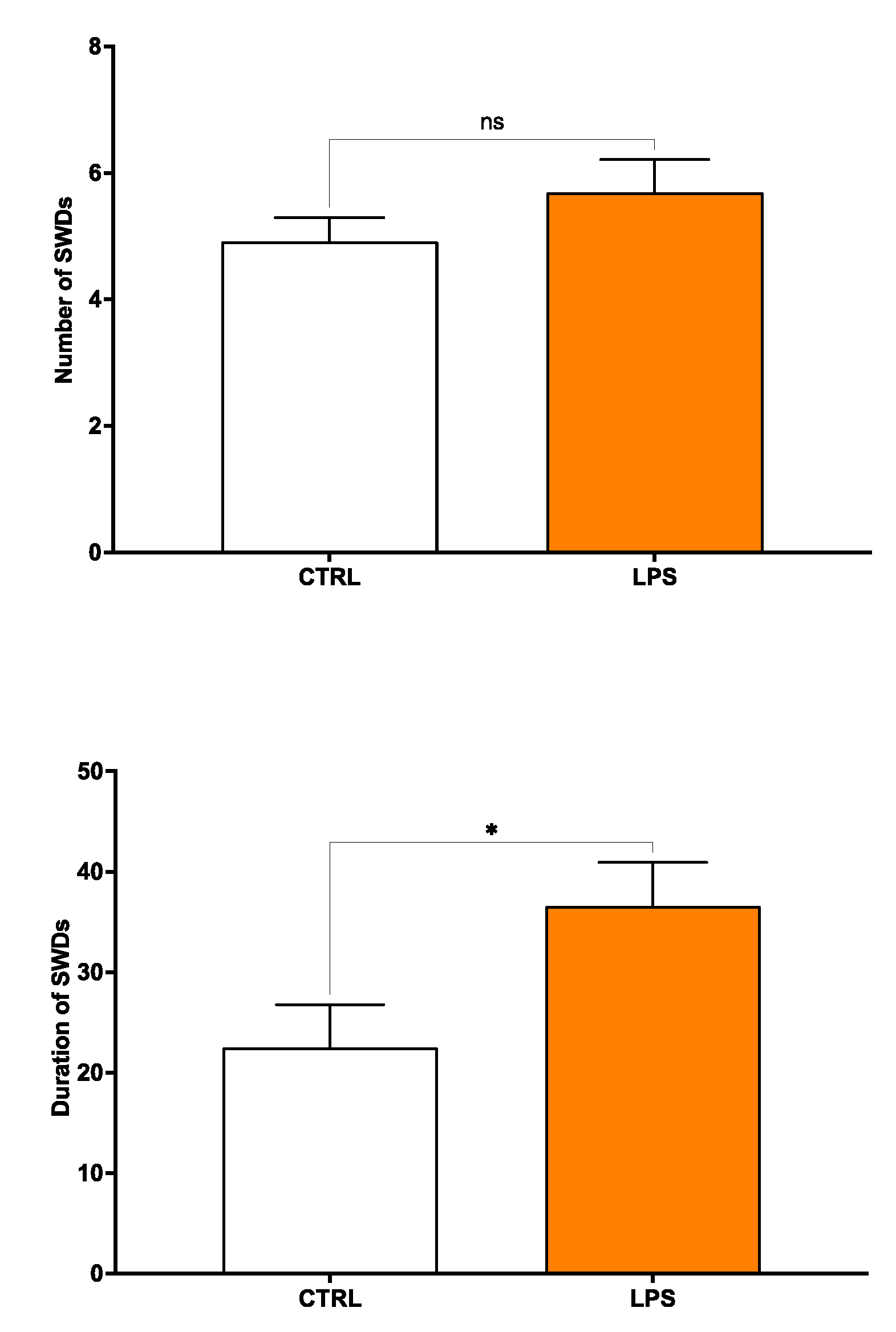
2.2. Early LPS Accelerates the Onset of Seizures in Young Adult Male WAG/Rij Rats
References
- Leo, V. Nesci, M. Tallarico, N. Amodio, E.M. Gallo Cantafio, G. De Sarro, A. Constanti, E. Russo, R. Citraro, IL-6 Receptor Blockade by Tocilizumab Has Anti-absence and Anti-epileptogenic Effects in the WAG/Rij Rat Model of Absence Epilepsy, Neurotherapeutics 17(4) (2020) 2004-2014.
- M. Tallarico, A. Leo, L. Guarnieri, M.C. Zito, C. De Caro, F. Nicoletti, E. Russo, A. Constanti, G. De Sarro, R. Citraro, N-acetylcysteine aggravates seizures while improving depressive-like and cognitive impairment comorbidities in the WAG/Rij rat model of absence epilepsy, Molecular Neurobiology 59(5) (2022) 2702-2714.
- Nakagaki, B.N.; Mafra, K.; de Carvalho, E.; Lopes, M.E.; Carvalho-Gontijo, R.; de Castro-Oliveira, H.M.; Campolina-Silva, G.H.; de Miranda, C.D.M.; Antunes, M.M.; Silva, A.C.C.; et al. Immune and metabolic shifts during neonatal development reprogram liver identity and function. J Hepatol 2018, 69, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Romics, L., Jr.; Frendl, G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis 2002, 6, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M. Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity. Front Immunol 2013, 4, 109. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Zhai, R.; Liang, H.; Song, G.; Yuan, Y.; Xu, Y.; Yan, Y.; Qiu, L.; Sun, T. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered 2022, 13, 4598–4609. [Google Scholar] [CrossRef] [PubMed]
- Hastings, K.L.; Green, M.D.; Gao, B.; Ganey, P.E.; Roth, R.A.; Burleson, G.R. Beyond Metabolism: Role of the Immune System in Hepatic Toxicity. Int J Toxicol 2020, 39, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Luo, L.; Wang, Q.; Yan, S.; Lin, J.; Li, D.; Cao, B.; Mei, H.; Ying, B.; Bin, L.; et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab Invest 2018, 98, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Markley, M.A.; Pierro, A.; Eaton, S. Hepatocyte mitochondrial metabolism is inhibited in neonatal rat endotoxaemia: Effects of glutamine. Clin Sci (Lond) 2002, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Brealey, D.; Singer, M. Mitochondrial Dysfunction in Sepsis. Curr Infect Dis Rep 2003, 5, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Oliveira, S. Neurologic manifestations of gastrointestinal and liver diseases. Curr Neurol Neurosci Rep 2014, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, D.; Solmaz, V.; Taskiran, D.; Erbas, O. The association between seizure predisposition and inflammation in a rat model of fatty liver disease. Neurol Sci 2014, 35, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, J.; Cui, P.; Zhou, Y.; Liu, C.; Wu, X.; Ji, Y.; Wang, S.; Cheng, B.; Ye, H.; et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect Immun 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat Rev Immunol 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression [corrected]. Prog Neuropsychopharmacol Biol Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; De Caro, C.; Citraro, R.; Russo, E.; Tallarico, M.; Iannone, M.; Ferrante, M.C.; et al. Butyrate prevents valproate-induced liver injury: In vitro and in vivo evidence. FASEB J 2020, 34, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased Metabolic Flexibility in Skeletal Muscle of Rat Fed with a High-Fat Diet Is Recovered by Individual CLA Isomer Supplementation via Converging Protective Mechanisms. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Cairns, C.B.; Walther, J.; Harken, A.H.; Banerjee, A. Mitochondrial oxidative phosphorylation thermodynamic efficiencies reflect physiological organ roles. Am J Physiol 1998, 274, R1376–R1383. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal Biochem 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Maurano, F.; Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic Biol Med 2007, 43, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Harbeson, D.; Francis, F.; Bao, W.; Amenyogbe, N.A.; Kollmann, T.R. Energy Demands of Early Life Drive a Disease Tolerant Phenotype and Dictate Outcome in Neonatal Bacterial Sepsis. Front Immunol 2018, 9, 1918. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr Res 2020, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Grayck, M.R.; McCarthy, W.C.; Solar, M.; Balasubramaniyan, N.; Zheng, L.; Orlicky, D.J.; Wright, C.J. Implications of neonatal absence of innate immune mediated NFkappaB/AP1 signaling in the murine liver. Pediatr Res 2024. [Google Scholar] [CrossRef] [PubMed]
- Zarate, M.A.; Nguyen, L.M.; De Dios, R.K.; Zheng, L.; Wright, C.J. Maturation of the Acute Hepatic TLR4/NF-kappaB Mediated Innate Immune Response Is p65 Dependent in Mice. Front Immunol 2020, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Dembek, A.; Laggai, S.; Kessler, S.M.; Czepukojc, B.; Simon, Y.; Kiemer, A.K.; Hoppstadter, J. Hepatic interleukin-6 production is maintained during endotoxin tolerance and facilitates lipid accumulation. Immunobiology 2017, 222, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.A.; Watson, G.S.; Montine, T.J.; Wang, Q.; Green, P.S.; Kulstad, J.J.; Cook, D.G.; Peskind, E.R.; Baker, L.D.; Goldgaber, D.; et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol 2005, 62, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Aomatsu, M.; Kato, T.; Kasahara, E.; Kitagawa, S. Gender difference in tumor necrosis factor-alpha production in human neutrophils stimulated by lipopolysaccharide and interferon-gamma. Biochem Biophys Res Commun 2013, 441, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.; Keel, M.; Zellweger, R.; Steckholzer, U.; Trentz, O.; Ertel, W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma 2000, 48, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Frink, M.; Pape, H.C.; van Griensven, M.; Krettek, C.; Chaudry, I.H.; Hildebrand, F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 2007, 27, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Knoferl, M.W.; Angele, M.K.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit Care Med 2002, 30, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Knoferl, M.W.; Angele, M.K.; Diodato, M.D.; Schwacha, M.G.; Ayala, A.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg 2002, 235, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence 2014, 5, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R., 2nd; Friedman, J.E. Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease. J Clin Invest 2018, 128, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wen, Y.; Li, J.; Zhang, H.; Liu, Y. Prenatal Lipopolysaccharide Exposure Promotes Dyslipidemia in the Male Offspring Rats. Front Physiol 2018, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 2005, 336, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bedoucha, M.; Atzpodien, E.; Boelsterli, U.A. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol 2001, 35, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Masih-Khan, E.; Lo, M.; van Breemen, C.; McManus, B.M.; Dube, G.P. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 2001, 224, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B., Jr.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 2003, 111, 737–747. [Google Scholar] [CrossRef]
- Matsui, J.; Terauchi, Y.; Kubota, N.; Takamoto, I.; Eto, K.; Yamashita, T.; Komeda, K.; Yamauchi, T.; Kamon, J.; Kita, S.; et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes 2004, 53, 2844–2854. [Google Scholar] [CrossRef]
- Tai, E.S.; bin Ali, A.; Zhang, Q.; Loh, L.M.; Tan, C.E.; Retnam, L.; El Oakley, R.M.; Lim, S.K. Hepatic expression of PPARalpha, a molecular target of fibrates, is regulated during inflammation in a gender-specific manner. FEBS Lett 2003, 546, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Ohhira, M.; Motomura, W.; Fukuda, M.; Yoshizaki, T.; Takahashi, N.; Tanno, S.; Wakamiya, N.; Kohgo, Y.; Kumei, S.; Okumura, T. Lipopolysaccharide induces adipose differentiation-related protein expression and lipid accumulation in the liver through inhibition of fatty acid oxidation in mice. J Gastroenterol 2007, 42, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Reddy, J.K. PPARalpha in the pathogenesis of fatty liver disease. Hepatology 2004, 40, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Hashimoto, T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu Rev Nutr 2001, 21, 193–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Sewal, R.K.; Modi, M.; Saikia, U.N.; Chakrabarti, A.; Medhi, B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res 2017, 135, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure 2012, 21, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert Rev Neurother 2018, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int J Mol Med 2024, 53. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, T.; Patel, M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front Cell Dev Biol 2022, 10, 976953. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. Pathophysiology of mitochondrial disease causing epilepsy and status epilepticus. Epilepsy Behav 2015, 49, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.P.; Waldbaum, S.; Rowley, S.; Huang, T.T.; Day, B.J.; Patel, M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol Dis 2012, 45, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv Clin Chem 2018, 87, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.C.; Zaretsky, M.D.; Dubs, J.G.; Roederer, M.; Anderson, M.; Green, A.; Mitra, D.; Watanabe, N.; Nakamura, H.; Tjioe, I.; et al. N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest 2000, 30, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




