Introduction
Intralabyrinthine schwannoma (ILS) is a well-known tumor of the lateral skull base occurring in various locations. They are most frequently found in the cochlea with a rate of about 60 % (Kennedy et al., 2004; Salzmann et al., 2012). Cochlear implantation is the well-accepted form of rehabilitation for hearing loss in these cases and was first described by Kronenberg (1999).
Related to the broadening of indication for cochlear implantation to the group of patients with single-sided deafness, the number of found ILS is increasing, which is underlined by a growing number of international publications (Francella et al., 2023).
The handling of the tumor itself is controversially discussed. The mean growth rate of the tumor is calculated as 0.25 mm per year (Khera et al., 2023) and hence is considered as low. However, the fact that the tumor does enlarge and single faster-growing cases are known contributes to the relevance of the debate revolving around the management of this tumor. The approaches were quite diverging. Some describe a cochlear implant insertion laterally, through the tumor without its removal (Carlson et al., 2016; Etuiis et al., 2021; Laborai et al., 2022) or after radiation (Carlson et al.,2016) but most groups extirpate the tumor before array placement. The surgical handling applied depends on the localization of the tumor. Should the tumor be covered by the modiolus, different techniques are known like a cochlectomy (Plontke et al., 2017; 2021), which includes revealing the top of the promontory, removal of the tumor, and covering the situs with cartilage after electrode placement. Another technique is the application of a Cochlear Advance Dummy electrode for an extended round window access and pushing the tumor through a second turn access (Aschendorf et al., 2017). A third technique consists of pushing dry gel-foam into the extended round window access and by this pushing the tumor out of the second turn access. Floating, upswelling of the gel-foam enables a removal by sucking the material out of the cochlea (Sudhoff et al., 2021). A fourth technique uses a dummy electrode by cutting the bulky basal part and pushing it in a turned manner to push the tumor out of the second turn access. A fifth technique is close to the second technique but uses a 28 mm stiffened lateral wall device (TRD), but until now just demonstrated in a human temporal bone model (Sudhoff et al., 2022). This technique showed, that it is easy to grab and remove the tumor by a by pipe- cleaner like technique.
Besides these surgical techniques, watch-and-wait or radio-surgical treatment approaches have been discussed and performed , especially in cases of NF II (Etuis et al., 2021).
As shown for central tumors, the MRI-based follow-up is highly important and well executable, even in cases with a cochlear implant, since the cochlear implant magnet artifacts are no longer an obstacle (Sudhoff et al., 2020). More precisely, implant and head positioning in the scanner, choice of MRI sequence, and implant magnet have been shown to allow a follow-up of VS (Sudhoff et al., 2020), ILS (Sudhoff et al., 2021), and glioma (Canzi et al., 2022) by MRI.
The present study aimed to describe the first experiences of a newly developed device for removing cochlear intra-labyrinthine schwannoma in vivo.
Material and Methods
Three patients who underwent ILS removal with a new TRD were included in this retrospective study. One patient is suffering from an NFII (ILS with combined persisting VS), and two with a pure intracochlear schwannoma, and an additional single-staged cochlear implantation.
The used tissue removal device (TRD) had the outer shape of a CI electrode array. It is a custom-made device by MEDEL Company (Innsbruck, Austria). It is mechanically stabilized, and two silicone rings are basally added with a diameter of 1.35 mm. These silicone rings are 1 mm apart and 0.5 mm in width (Sudhoff et al., 2023).
On the day after surgery, an MRI scan was performed to evaluate residual tumor persistence with a 3T MRI scanner (Philips, Acheiva) by applying the T1 KM sequence.
The study was approved by the Ethical Committee of the University of Münster (2023 689 f-S).
Results
In all cases, a regular surgical procedure to remove cochlear ILS through a posterior tympanotomy after removal of the incus was performed. An enlarged cochleostomy was burred, and additional access through the posterior tympanotomy to the second turn of the cochlea was introduced about 1.5 mm caudally from the facial nerve and 1 mm above the oval window, as described by others.
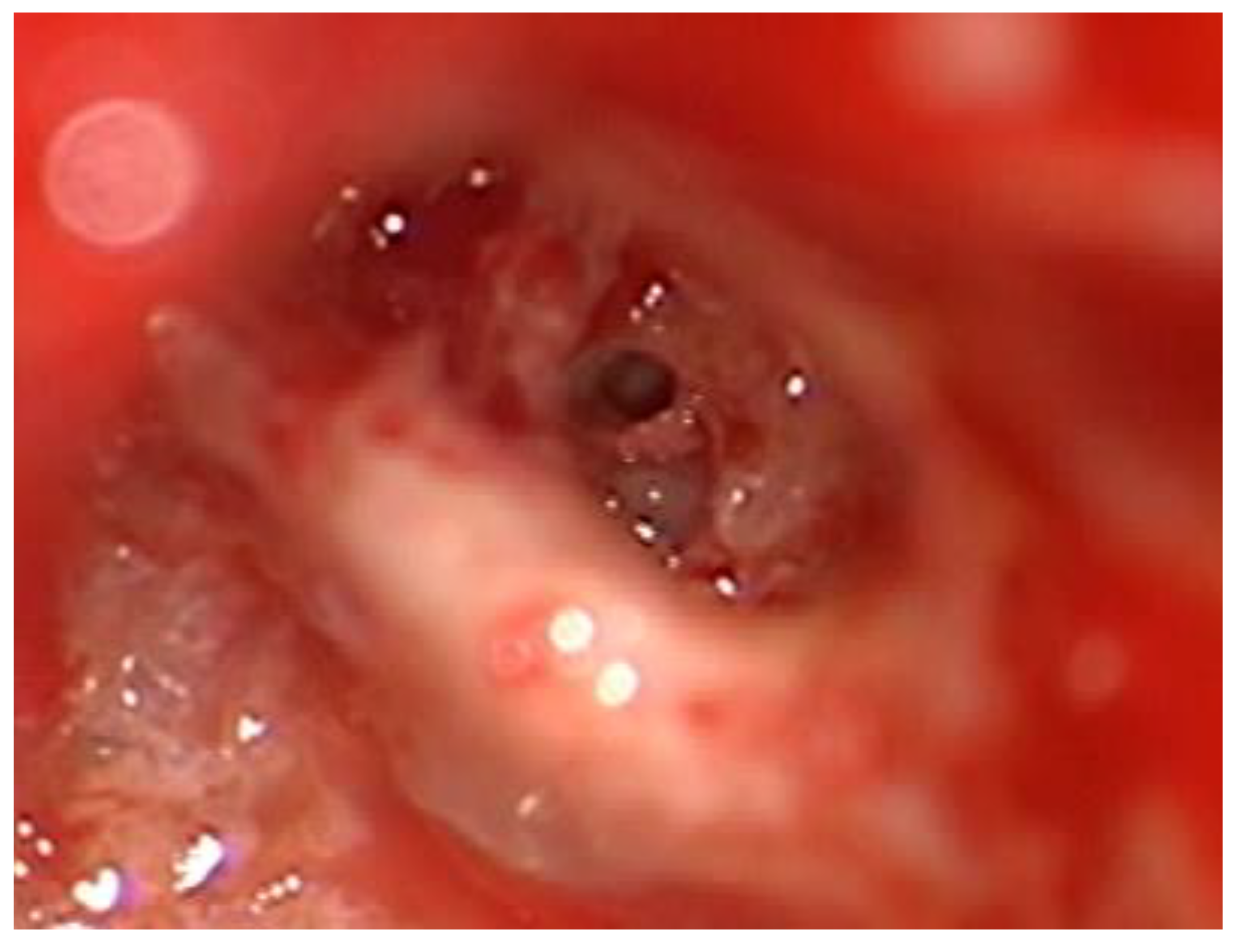
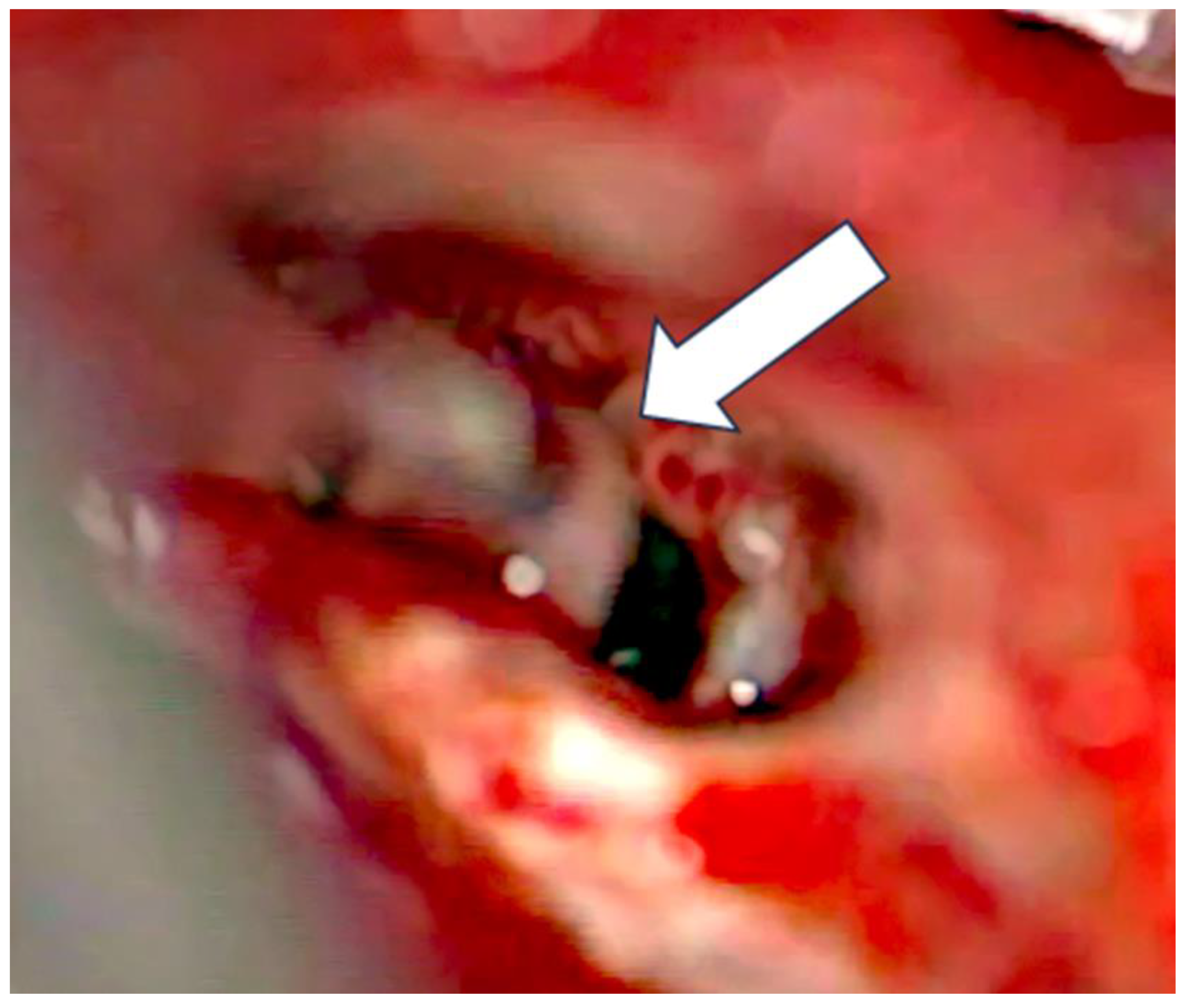
After the removal of parts of the tumor through the enlarged cochleostomy (
Figure 1) the TRD was introduced (
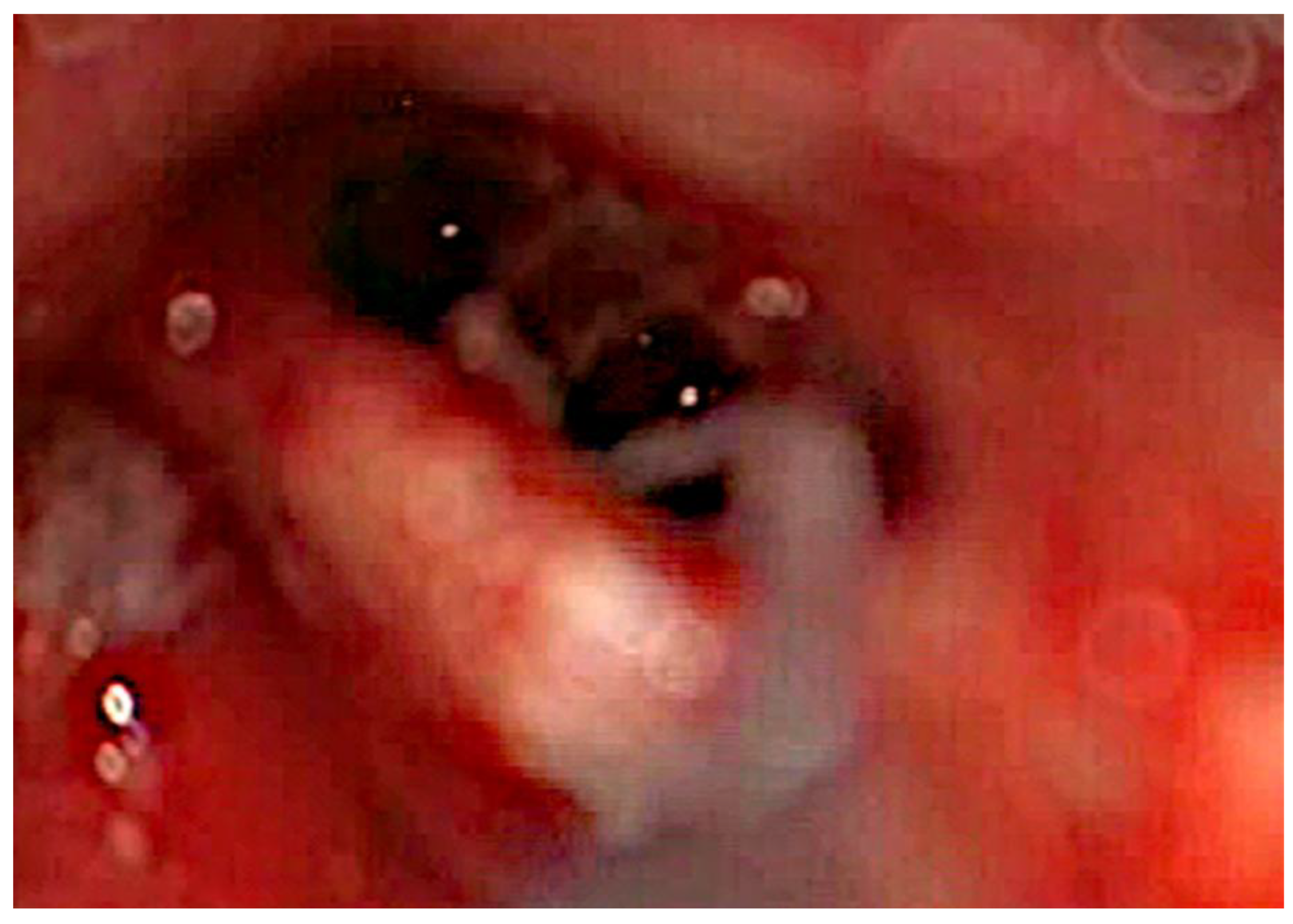
Figure 2) until the tip was visualized (
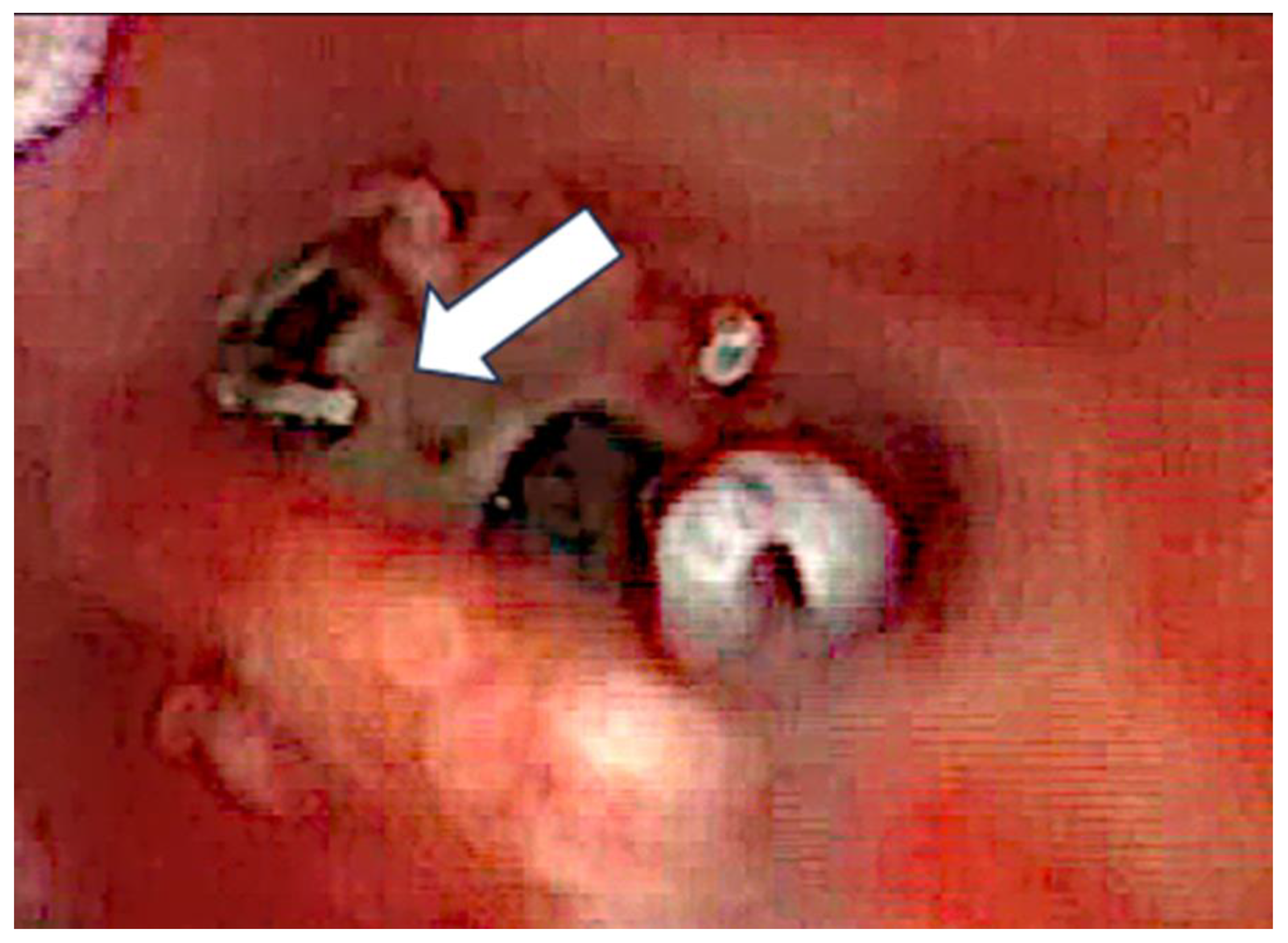
Figure 3) and carefully grabbed out of the second turn access. By grabbing the tip, the device slipped into the cochleostomy and pushed parts of the tumor out of the second turn. Residual tumor parts could be detached by pipe cleaner handling (
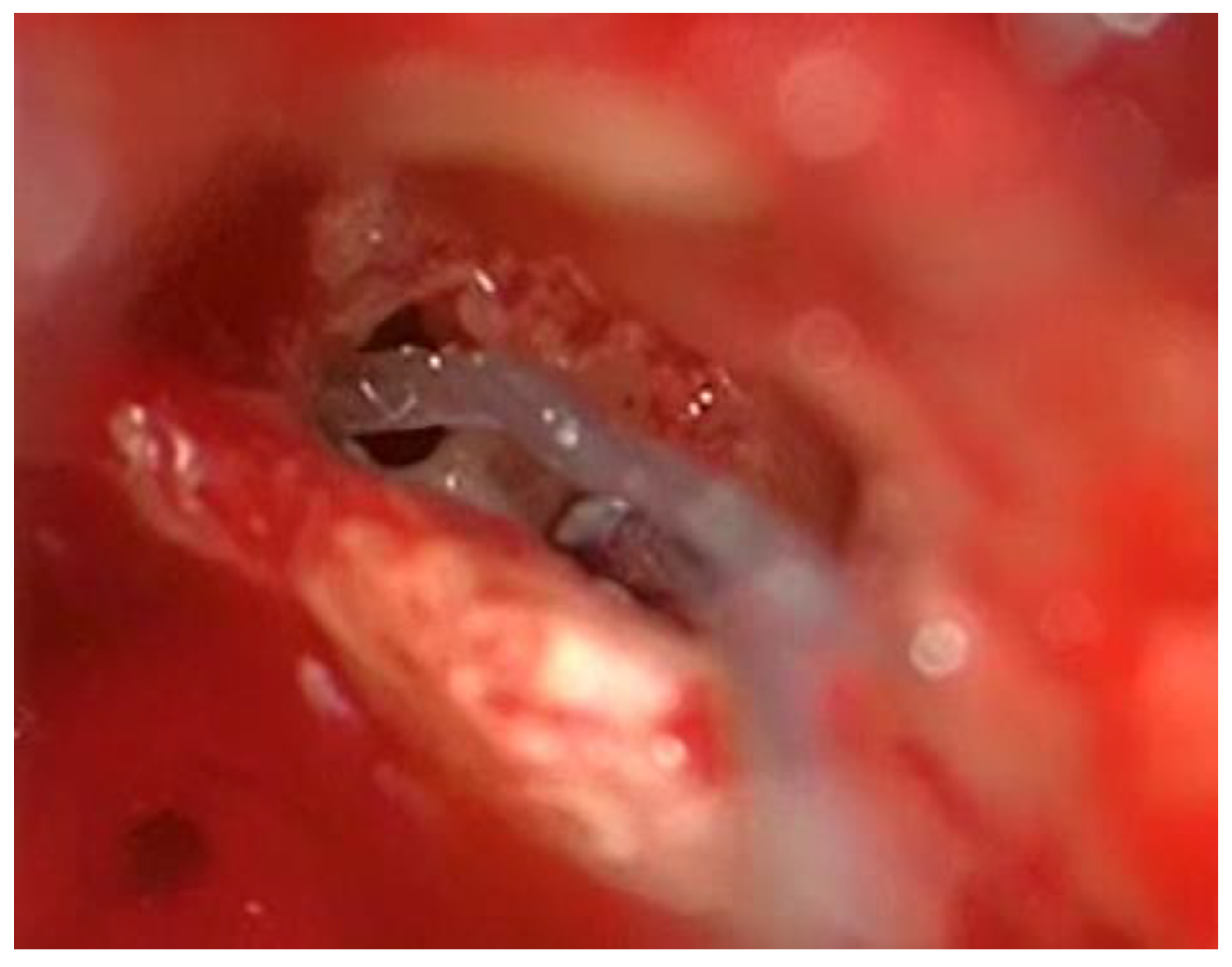
Figure 4) and sucked out (
Figure 5).
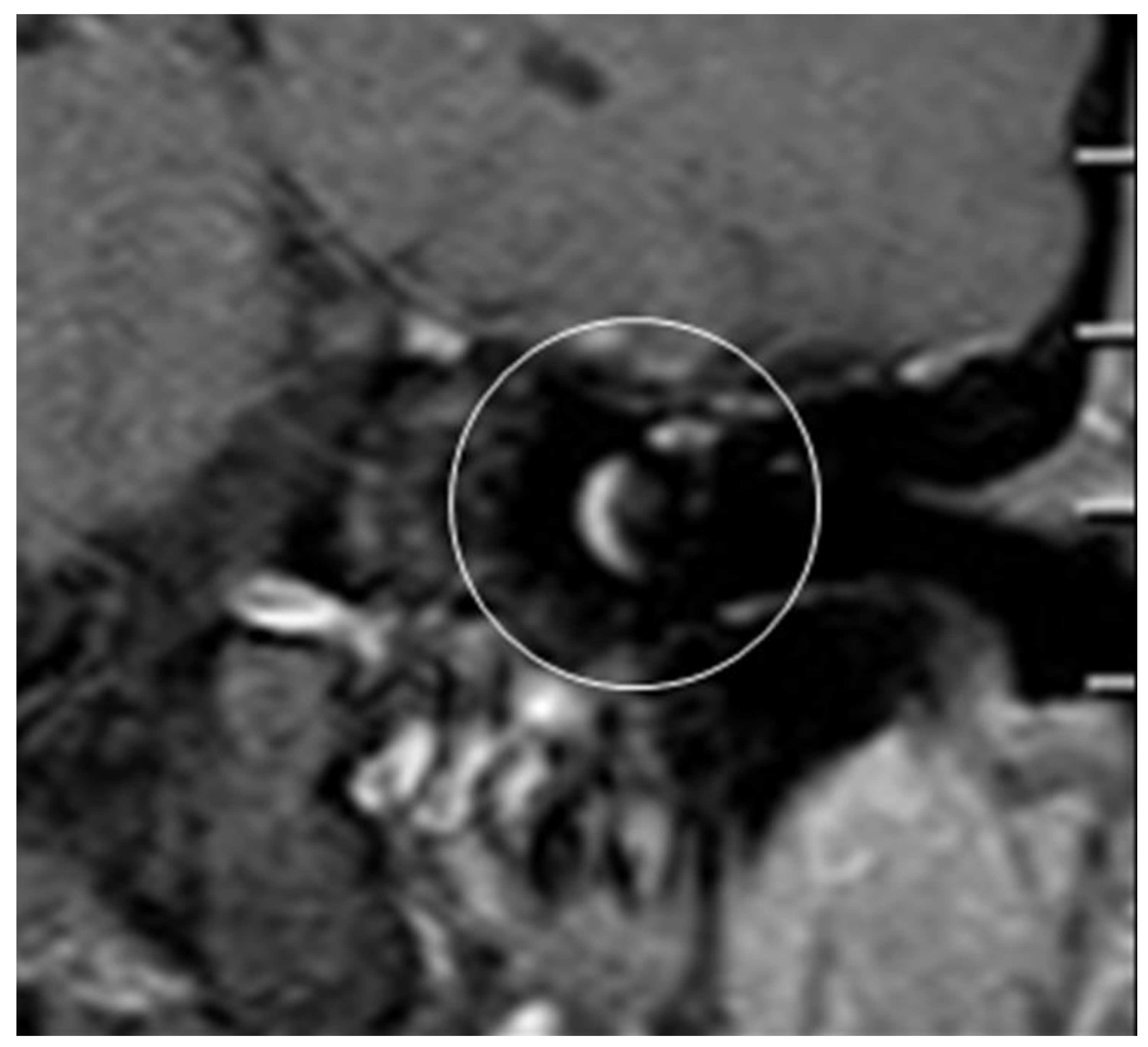
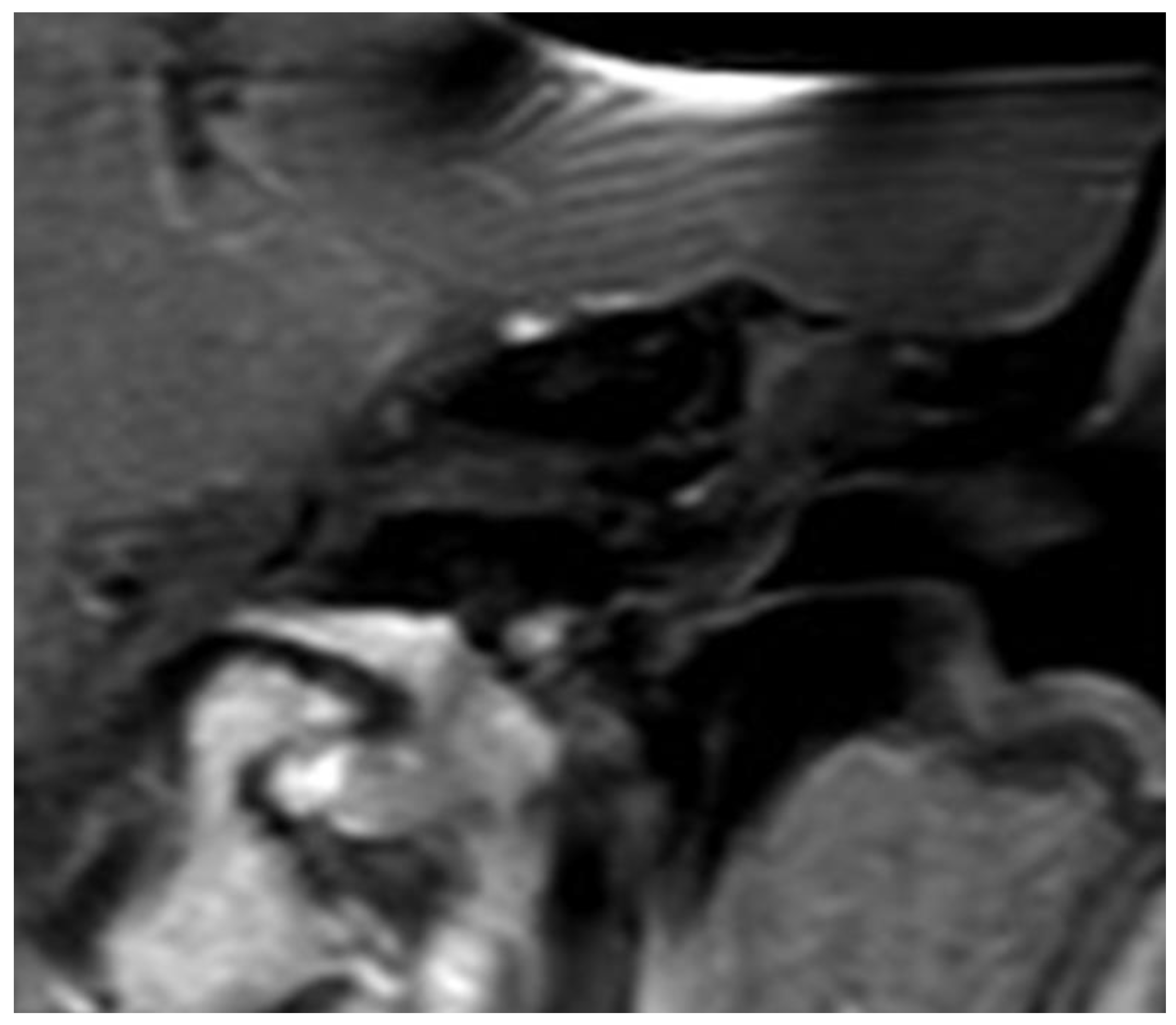
The whole procedure lasted 15 minutes in each case. Comparing pre-OP (
Figure 6) and post-OP (
Figure 7) MRI scanning confirmed a complete removal of the tumor.
Discussion
Intra-labyrinthine schwannoma are tumors of the lateral skull base that can occur as part of an NFII or spontaneously.
In many cases, the accompanying hearing loss is restored by cochlear implantation. Apart from the concept of a non-removal of the tumor applied in some centers by pushing the cochlear implant electrode through the tumor (Carlson, Etuis, Labori), the ILS is mostly removed to prevent the risk of tumor growth. Recently, we described a new device for the removal of cochlear schwannoma (Sudhoff et al., 2022) in human temporal bone models.
The proposed handling of the device was now verified in the first three in vivo observations. Similar to the observations made in the temporal bone model, the tumor could be removed by a combination of an enlarged cochleostomy and a second-turn access. The proposed pushing and pipe cleaner handling by grabbing the tip allows a straightforward performance of the removal. In both cases, the whole procedure of tumor removal lasted 15 min and the following MRI scan confirmed a complete removal of the tumor.
Comparing this technique with others, it is important to mention that the audiological outcome of cochlear implantation in ILS cases allows a regular speech audiometric outcome in most cases (Jia et al., 2020). Therefore, handling, time, ease of procedure, and completeness of removal are in the focus.
While a cochlectomy is a time-consuming procedure (Plontke et al., 2017; 2021) it is indicated in cases, which need broader visual access to the situs than a pipe cleaner procedure. Comparing the proposed TRD handling with the usage of a Cochlear Advance dummy electrode (Aschendorf et al., 2017), which is quite close to our handling, the latter electrode is in our experience bulkier, and softer and the tip is more difficult to grip. The smaller size of the TRD positioned laterally in the cochlear scala enabled it to slip out of the second turn access, we assume this procedure to be less traumatic.
The described use of dry gel foam to push the tumor out of the second turn of the cochlea is, compared to the proposed technique, time-consuming and therefore less favorable (Sudhoff et al., 2021).
The general question of removing or not removing these tumors can easily be answered by the question of why not remove if you can minimize the risk of a growing tumor by a swift procedure. Future experiences will show if this array could be even used in cases of cochlea granulations apart from schwannoma. Here cases of intracochlear granulation after removed electrodes could be assumed.
Limitations of this study are the small number of patients, which is related to the low occurrence rate of these tumors, and the short follow-up period by the MRI scan. Since these tumors are regularly monitored, we will be able to compare different techniques in terms of their tumor control in the future.
Conclusion
In vivo, the handling of the TRD confirmed a straightforward handling for cochlear schwannoma removal. MRT scanning showed in all cases a complete removal of the tumor by the TRD.
Availability of data and materials
The data used to support this study’s findings are available from the corresponding author upon request.
Competing interest
The authors declare that they have no competing interests.
Author Contributions
PCJ analysis, correction, final approval; RC analysis, correction, final approval; KR analysis, correction, final approval; SLU analysis, correction, final approval; SM Analysis, correction, final approval; TI writing, idea, correction, final approval.
Ethical approval: The study was approved by the Ethical Committee of the University of Münster (2023 689 f-S).
Acknowledgments
MEDEL: Innsbruck, Austria kindly provided custom-made devices.
References
- 1. Kennedy R, Shelton C, Salzman K, et al., Intralabyrinthine schwannomas: diagnosis, management, and a new classification system. Otol Neurotol. 2004;25:160-167.
- 2. Salzman KL, Childs AM, Davidson HC, et al., Intralabyrinthine schwannomas: imaging diagnosis and classification. Am J Neuroradiol, 2012;33(1):104-109. [CrossRef]
- 3. Kronenberg J, Horowitz Z, Hildesheimer M. Intracochlear schwannoma and cochlear implantation. Ann Otol Rhinol Laryngol, 1999;108:659-660.
- Franchella S, Ariano M, Bevilacqua F, Concheri S, Zanoletti E. Cochlear Implantation in Intralabyrinthine Schwannoma: Case Series and Systematic Review of the Literature. Audiol Res. 2023 Feb 28;13(2):169-184. [CrossRef]
- Khera Z, Kay-Rivest E, Friedmann DR, McMenomey SO, Thomas Roland J Jr, Jethanamest D. The Natural History of Primary Inner Ear Schwannomas: Outcomes of Long-Term Follow-Up. Otol Neurotol. 2022 Dec 1;43(10):e1168-e1173. [CrossRef] [PubMed]
- 6. Carlson ML, Neff BA, Sladen DP, et al., Cochlear implantation in patients with intracochlear and intralabyrinthine schwannomas. Otol Neurotol, 2016;37(6):647-653. [CrossRef]
- Eitutis Susan T, Jansen T, Borsetto D; et al., Cochlear Implantation in NF2 Patients Without Intracochlear Schwannoma Removal, Otol Neurotol. August 2021 - Volume 42 - Issue 7 - p1014-1021. [CrossRef]
- Laborai A, Ghiselli S, Cuda D. Cochlear Implant in Patients with Intralabyrinthine Schwannoma without Tumor Removal. Audiol Res. 2022 Jan 10;12(1):33-41. [CrossRef]
- 9. Plontke SK, Rahne T, Pfister M, et al. Intralabyrinthine schwannomas: surgical management and hearing rehabilitation with cochlear implants. HNO, 2017;65(Suppl 2):136-148. [CrossRef]
- Plontke, SK. An improved technique of subtotal cochleoectomy for removal of intracochlear schwannoma and single-stage cochlear implantation. Otol Neurotol, 2020;41(7):e891. [CrossRef]
- 11. Aschendorff A, Arndt S, Laszig R, et al. Treatment and auditory rehabilitation of intralabyrinthine schwannoma by means of cochlear implants: English version. HNO.2017;65(Suppl 1):46-51.
- Sudhoff H, Gehl HB, Scholtz LU, Todt I. MRI Observation After Intralabyrinthine and Vestibular Schwannoma Resection and Cochlear Implantation. Front Neurol. 2020 Aug 12;11:759. [CrossRef]
- Sudhoff H, Scholtz LU, Gehl HB, et al., Quality Control after Intracochlear.
- Intralabyrinthine Schwannoma Resection and Cochlear Implantation. Brain Sci. 2021 Sep 16;11(9):1221. [CrossRef]
- Canzi P, Luzzi S, Carlotto E, Simoncelli A, Brondino N, Marconi S, Magnetto M, Lucifero GA, Avato I, Manfrin M, Auricchio F, Preda L, Benazzo M. Customized Cochlear Implant Positioning in a Patient With a Low- Grade Glioma: Towards the Best MRI Artifact Management. Otol Neurotol. 2022 Jul 1;43(6):e628-e634. [CrossRef] [PubMed]
- Sudhoff H, Riemann C, Kim R, Scholtz LU, Pfeiffer CJ, Goon P, Todt I. A new device for the removal of cochlear
schwannoma: A temporal bone study. Front Surg. 2023 Feb 1;10:1077407. doi: 10.3389/fsurg.2023.1077407.
Erratum in: Front Surg. 2023 Apr 28;10:1195473. PMID: 36816011; PMCID: PMC9928970.
- Jia H, Nguyen Y, Hochet B et al.. NF2-Related Intravestibular Schwannomas: Long-Term Outcomes of Cochlear Implantation. Otol Neurotol. 2020 Jan;41(1):94-99. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).