Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
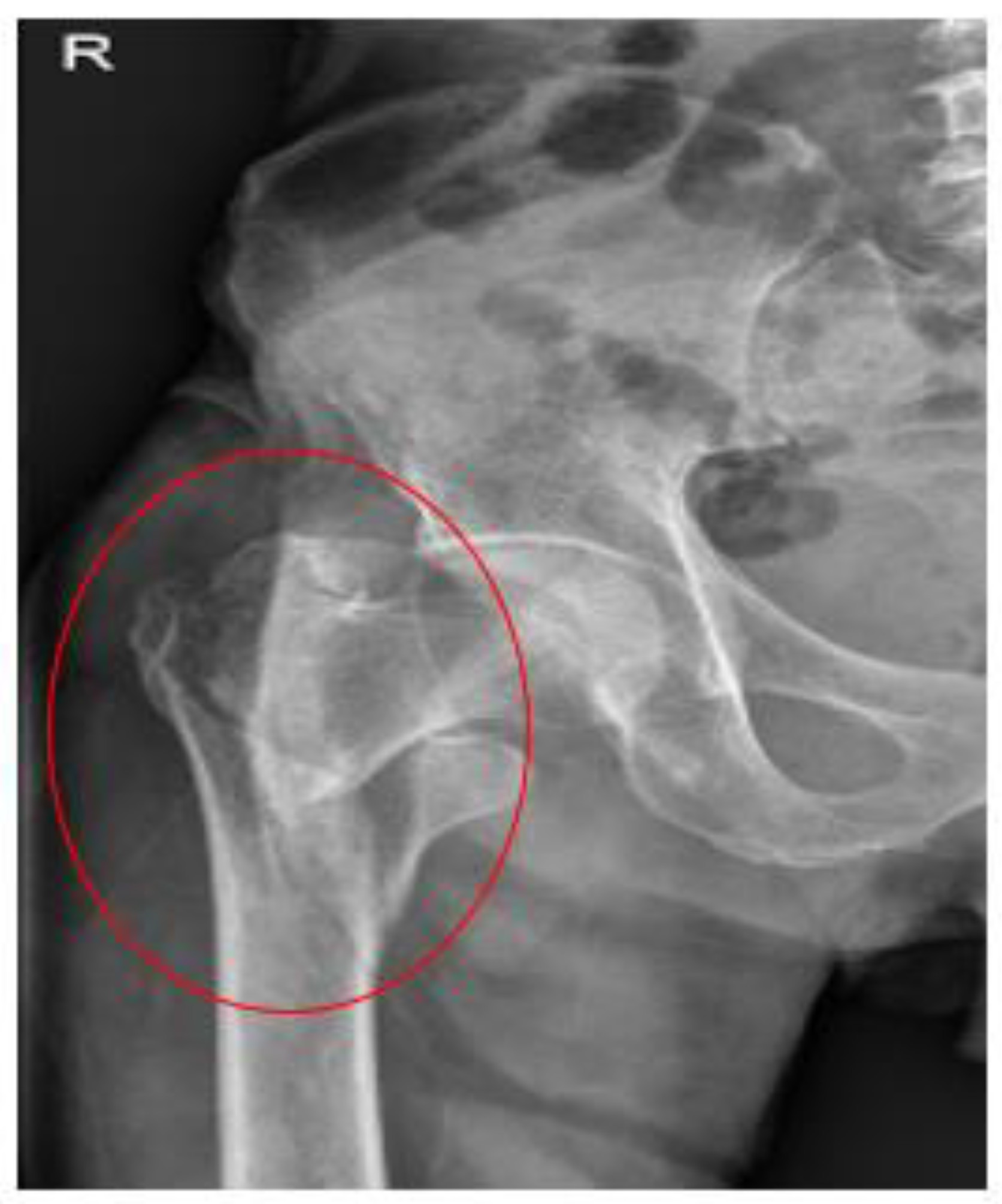
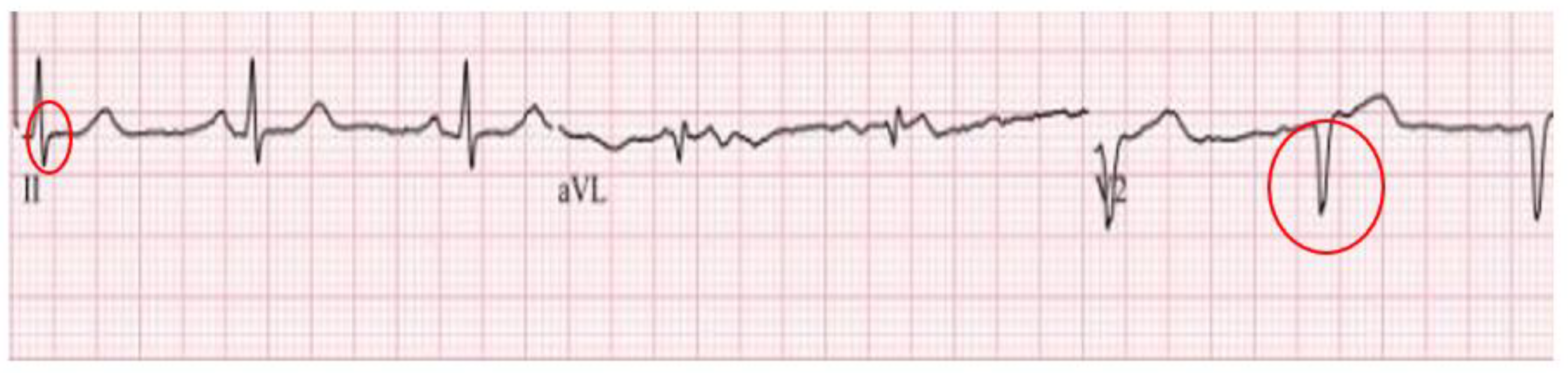
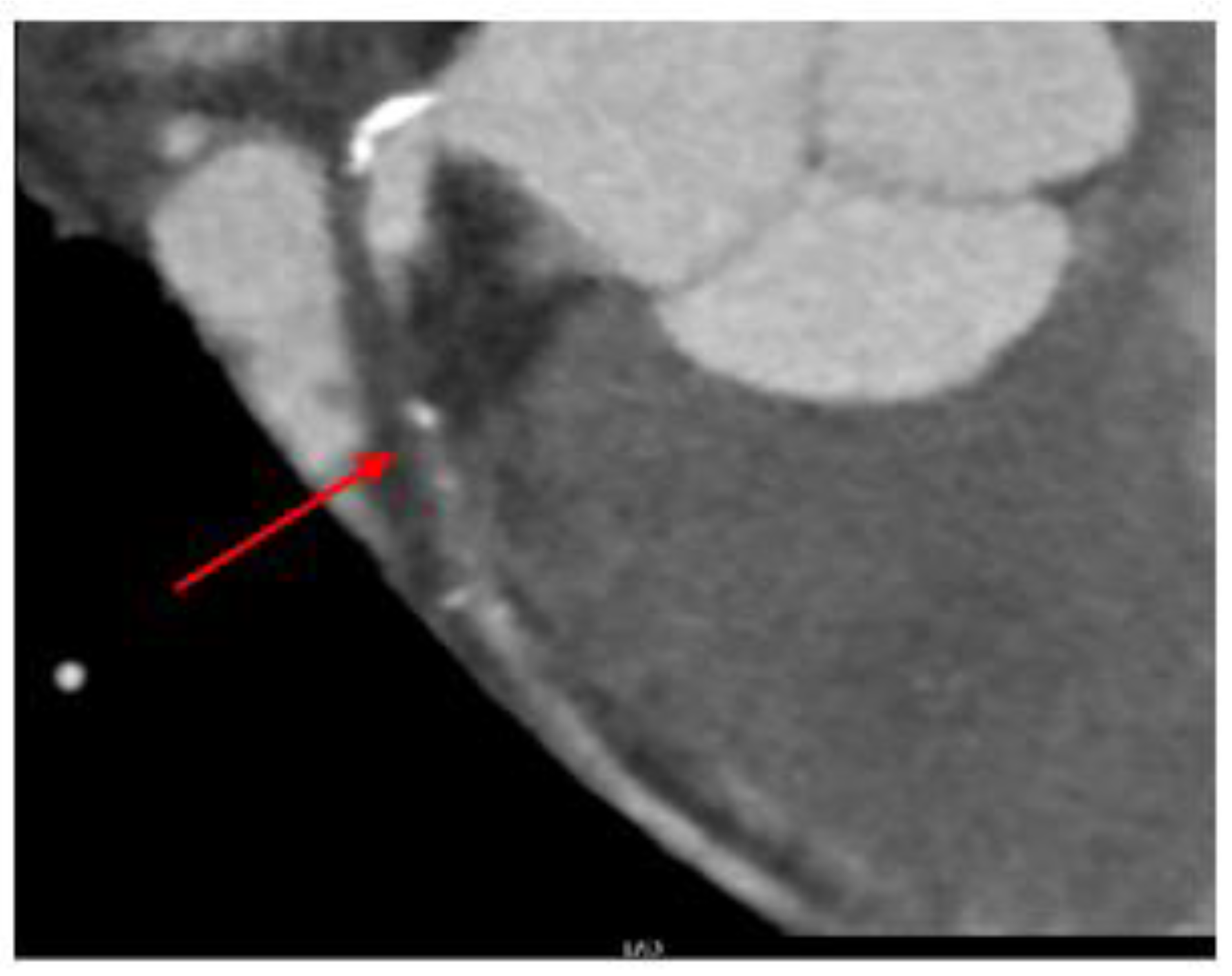
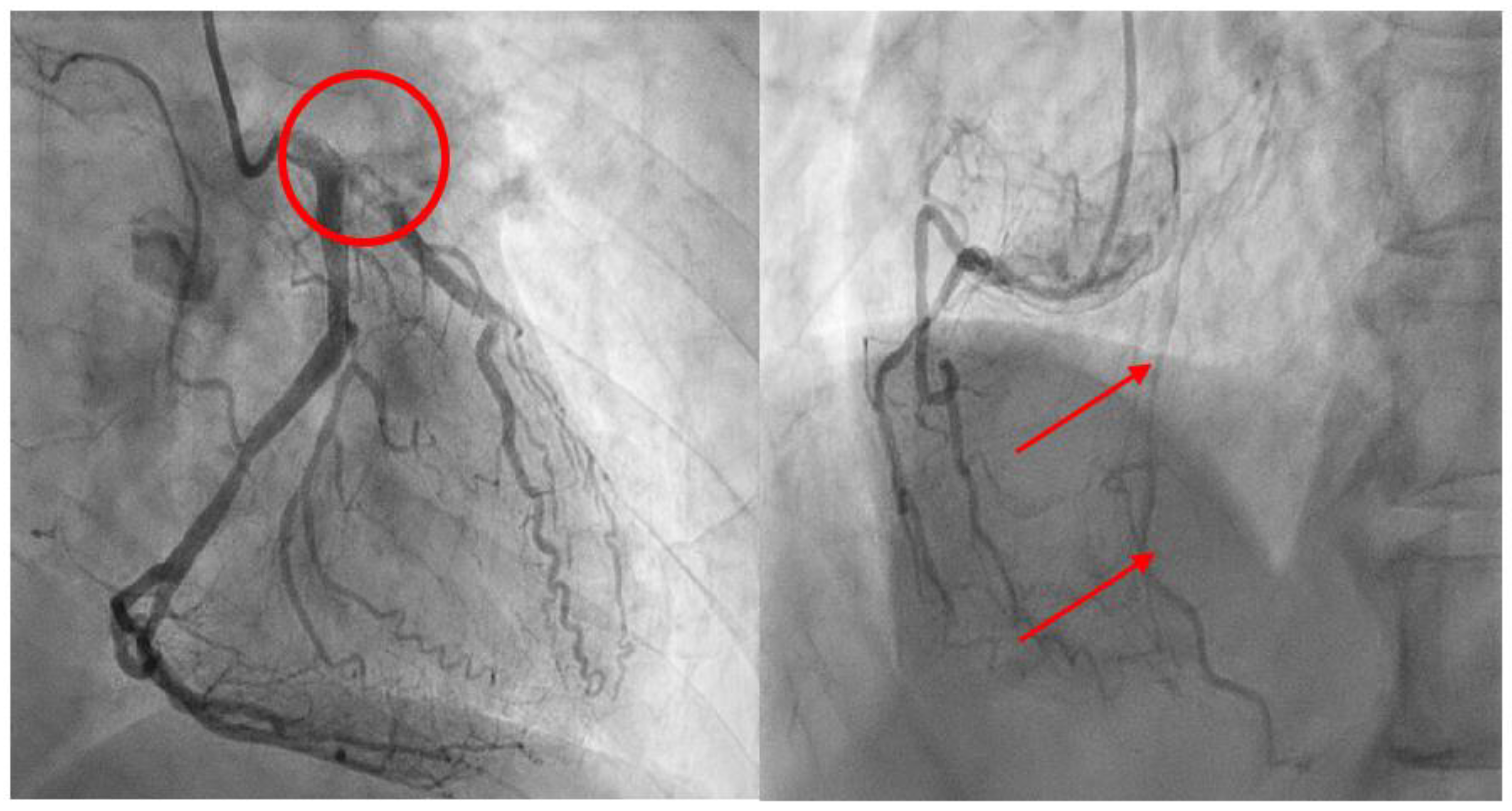
Case Report
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai ZL, Cai XT, Gao WL, Lin M, Lin J, Jiang YX, et al. Etomidate vs propofol in coronary heart disease patients undergoing major noncardiac surgery: A randomized clinical trial. World J Clin Cases. 2021;9:1293-303. [CrossRef]
- Ludders, JW. Anesthesia for patients with dehydration/hypovolemia. Vet Clin North Am Small Anim Pract. 1992;22:495-6. [CrossRef]
- Khalid L, Dhakam SH. A review of cardiogenic shock in acute myocardial infarction. Curr Car diol Rev. 2008;4:34-40. [CrossRef]
- Martin TC, Bhaskar YG, Umesh KV. Sensitivity and specificity of the electrocardiogram in pre dicting the presence of increased left ventricular mass index on the echocardiogram in Afro-Caribbean hypertensive patients. West Indian Med J. 2007;56:134-8. [CrossRef]
- Yang H, Deng HM, Chen HY, Tang SH, Deng F, Lu YG, et al. The impact of age on propofol requirement for inducing loss of consciousness in elderly surgical patients. Front Pharmacol. 2022;13:739552. [CrossRef]
- Flegal MC, Fox LK, Kuhlman SM. Principles of anesthesia monitoring and electrocardiogram. J Invest Surg. 2009;22:316-7. [CrossRef]
- Noel-Morgan J, Muir WW. Anesthesia-associated relative hypovolemia: mechanisms, moni tor ing, and treatment considerations. Front Vet Sci. 2018;5:53. [CrossRef]
- Wolff CB, Green DW. Clarification of the circulatory patho-physiology of anaesthesia – Im pli cations for high-risk surgical patients. Int J Surg. 2014;12:1348-56. [CrossRef]
- Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quan tifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441-53. [CrossRef]
- Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology. 2019;130:825-32. [CrossRef]
- Farhan M, Hoda MQ, Ullah H. Prevention of hypotension associated with the induction dose of propofol: A randomized controlled trial comparing equipotent doses of phenylephrine and ephedrine. J Anaesthesiol Clin Pharmacol. 2015;31:526-30. [CrossRef]
- Soh S, Song JW, Choi N, Shim J-K. Anesthetic-induced myocardial protection in cardiac sur gery: relevant mechanisms and clinical translation. APM. 2018;13:1-9. [CrossRef]
- Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Medicine. 2013;11:143. [CrossRef]
- Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation. 1991;83:739-46. [CrossRef]
- Pérez-Castellano N, García EJ, Abeytua M, Soriano J, Serrano JA, Elízaga J, et al. Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am Coll Cardiol. 1998;31:512-8. [CrossRef]
- Kaul, S. Assessment of myocardial collateral blood flow with contrast echocardiography. Ko rean Circ J. 2015;45:351-6. [CrossRef]
- Saraon T, Chadow HL, Castillo R. The power of collateral circulation: a case of asymptomatic chronic total occlusion of the left main coronary artery. J Invasive Cardiol. 2012;24:E196-8.
- Fadah K, Hechanova A, Mukherjee D. Epidemiology, pathophysiology, and management of cor onary artery disease in the elderly. Int J Angiol. 2022;31:244-50. [CrossRef]
- Gilstrap LG, Bhatia RS, Weiner RB, Dudzinski DM. Dobutamine stress echocardiography: a review and update. Res Rep Clin Cardiol. 2014;5(null):69-81. [CrossRef]
- Poldermans D, Fioretti PM, Forster T, Thomson IR, Boersma E, el-Said EM, et al. Dobutamine stress echocardiography for assessment of perioperative cardiac risk in patients undergoing major vascular surgery. Circulation. 1993;87:1506-12. [CrossRef]
- Kurdi MS, Theerth KA, Deva RS. Ketamine: Current applications in anesthesia, pain, and crit ical care. Anesth Essays Res. 2014;8:283-90. [CrossRef]
- Valk BI, Struys M. Etomidate and its analogs: a review of pharmacokinetics and pharmacody namics. Clin Pharmacokinet. 2021;60:1253-69. [CrossRef]
- Jakobsen CJ, Torp P, Vester AE, Folkersen L, Thougaard A, Sloth E. Ketamine reduce left ven tricular systolic and diastolic function in patients with ischaemic heart disease. Acta Anaesthesiol Scand. 2010;54:1137-44. [CrossRef]
- Kawasaki S, Kiyohara C, Tokunaga S, Hoka S. Prediction of hemodynamic fluctuations after induction of general anesthesia using propofol in non-cardiac surgery: a retrospective cohort study. BMC Anesthesiol. 2018;18:167. [CrossRef]
- Kim SH, Lee SH, Shim SH, Kim JS, Kwak SD, Kim CS, et al. Effects of etomidate, propofol and thiopental sodium on intraocular pressure during the induction of anesthesia. Korean J Anesthesiol. 2000;39:309-13. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




