Submitted:
23 August 2023
Posted:
24 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
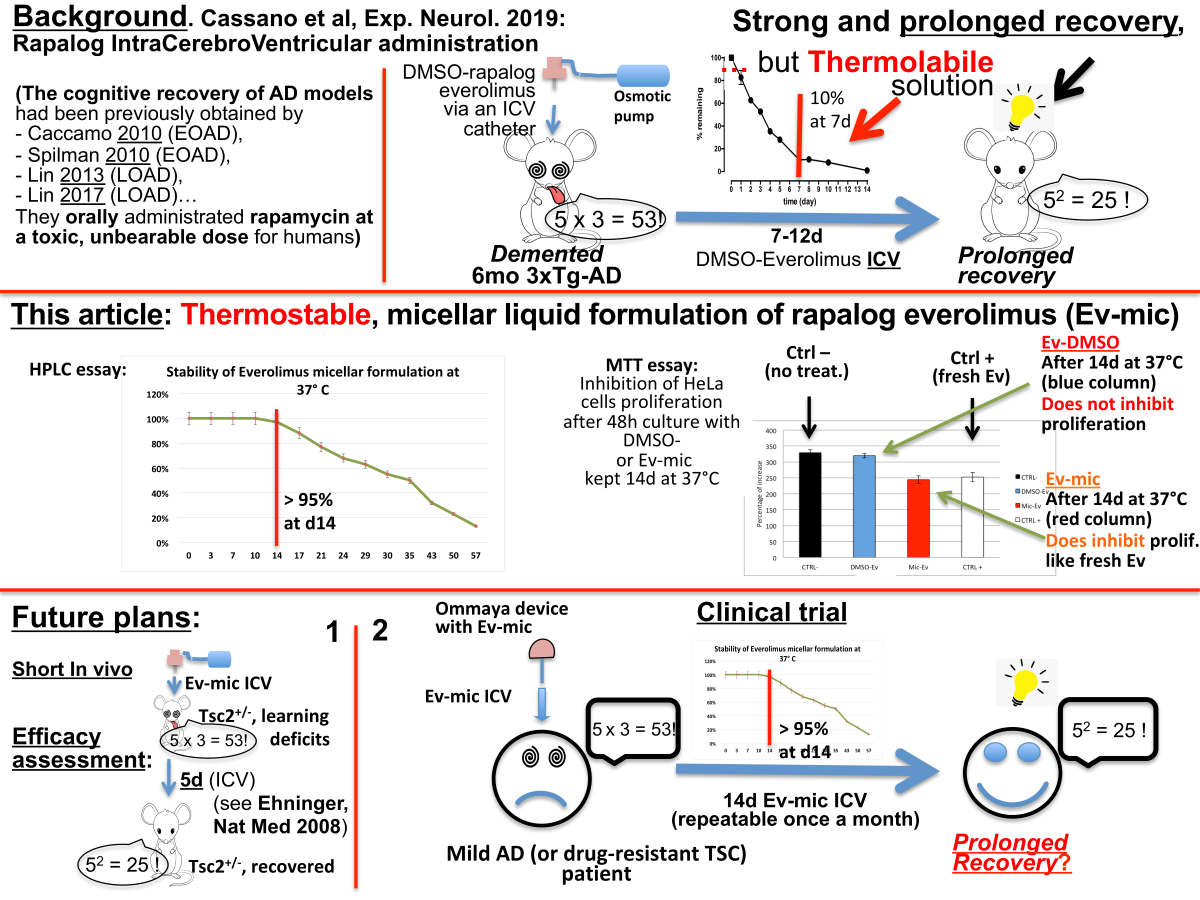
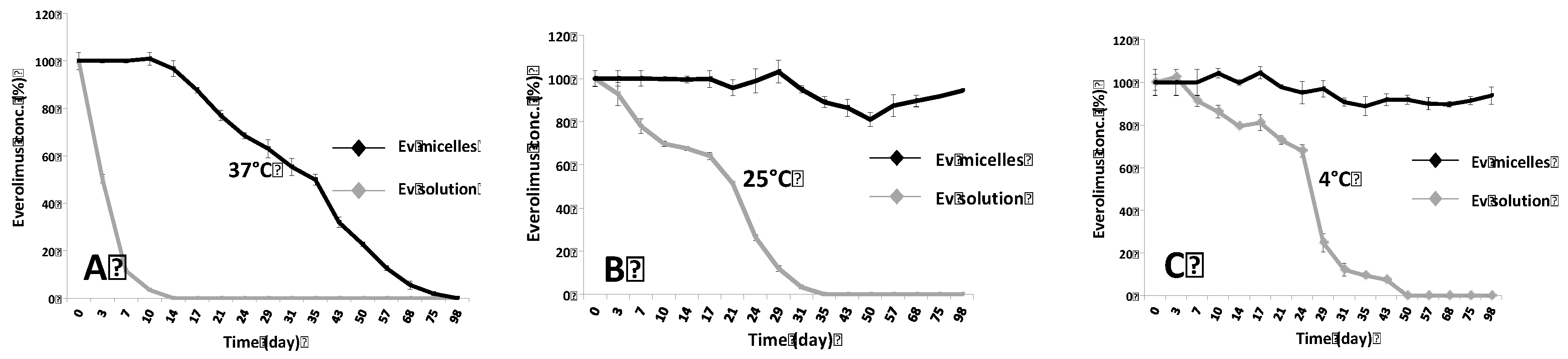
2.1. Evaluation of Ev-Sol and Ev-Mic Stability by HPLC Analysis
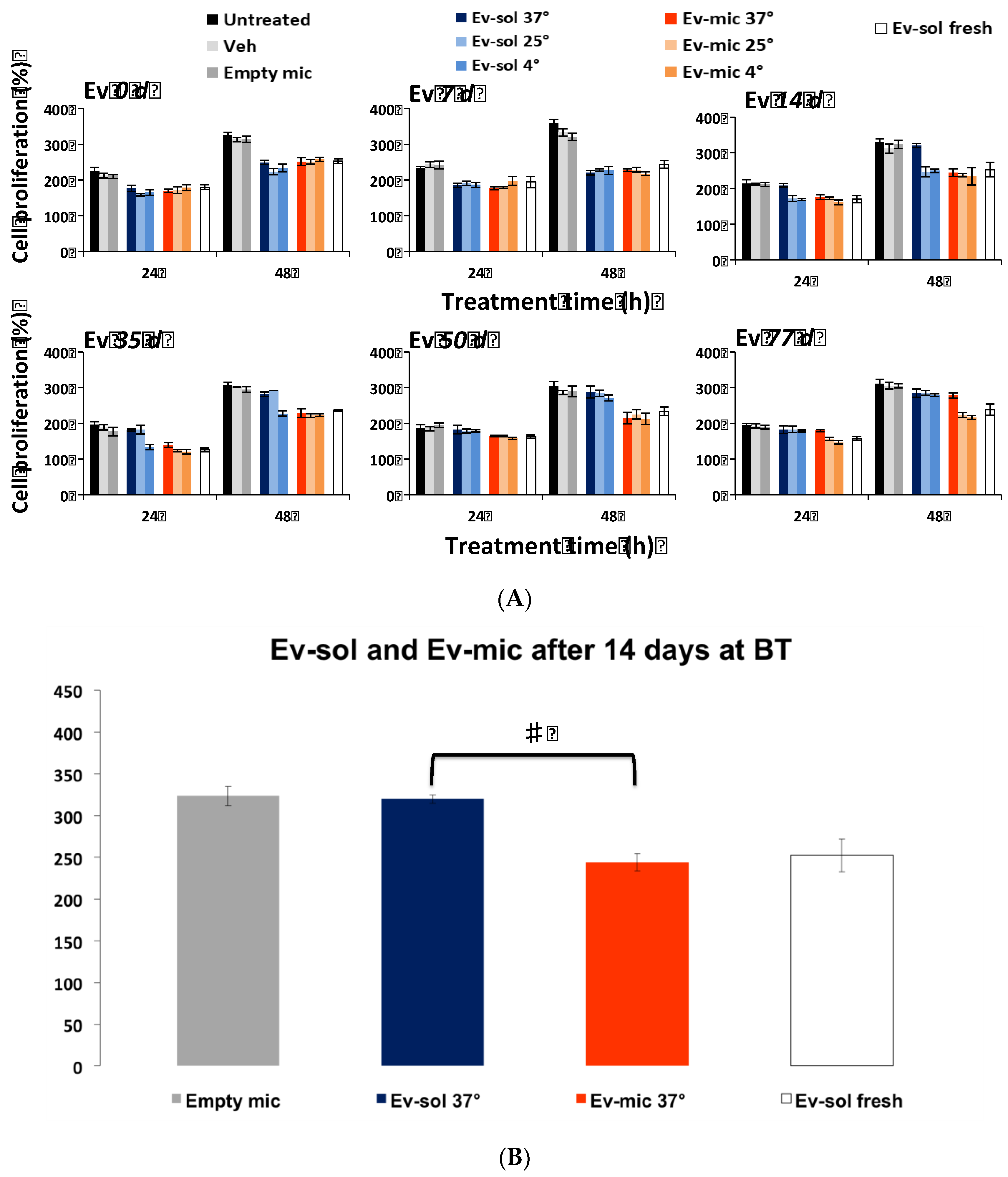
2.2. Evaluation of Ev-sol and Ev-mic Stability by Cell Cultures
2.3. Empty Micelles Toxicity was Evaluated by MTT Analysis
3. Discussion
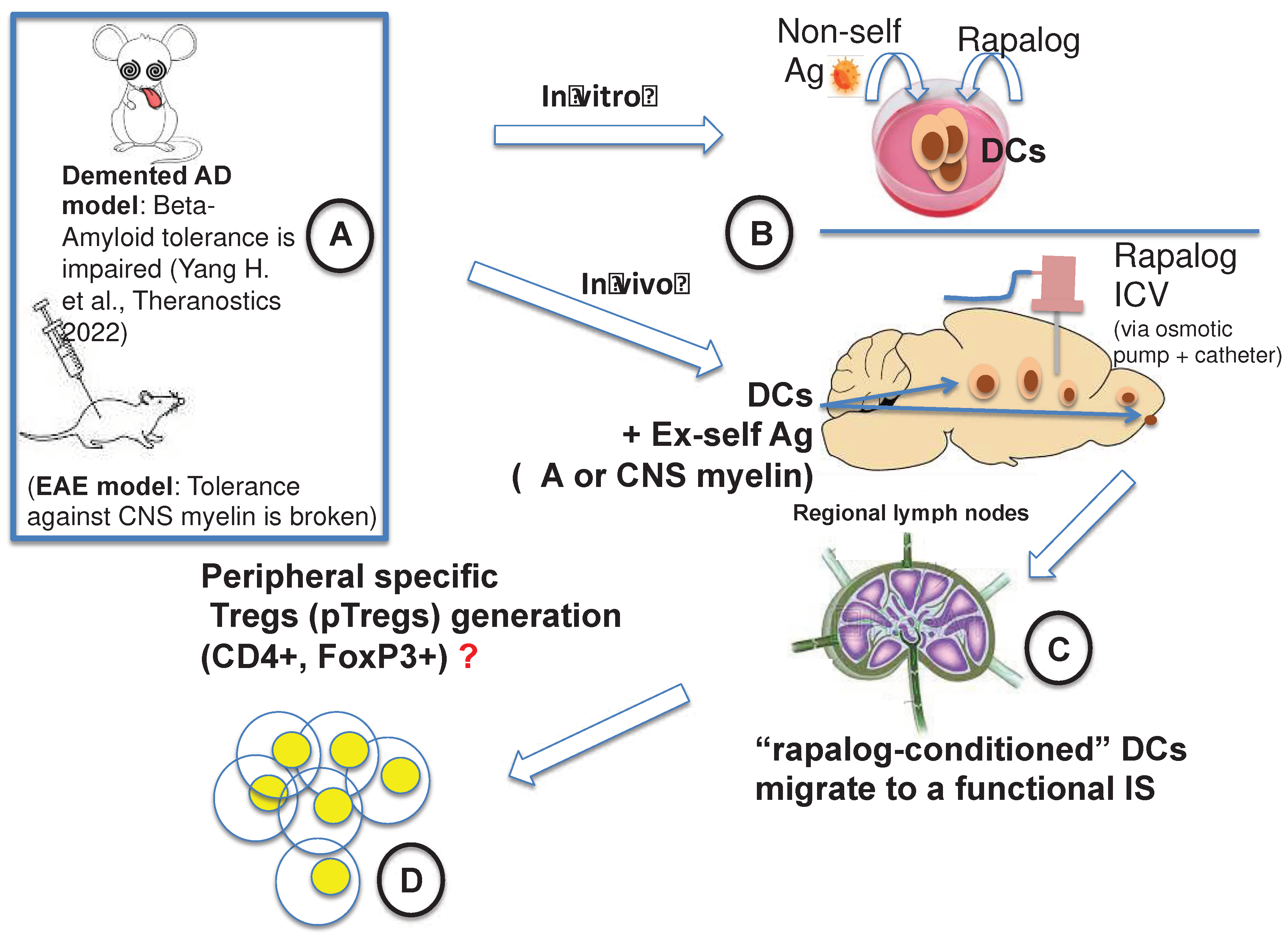
3.1. Our Attempt to Make the mTOR Inhibition in AD Translational
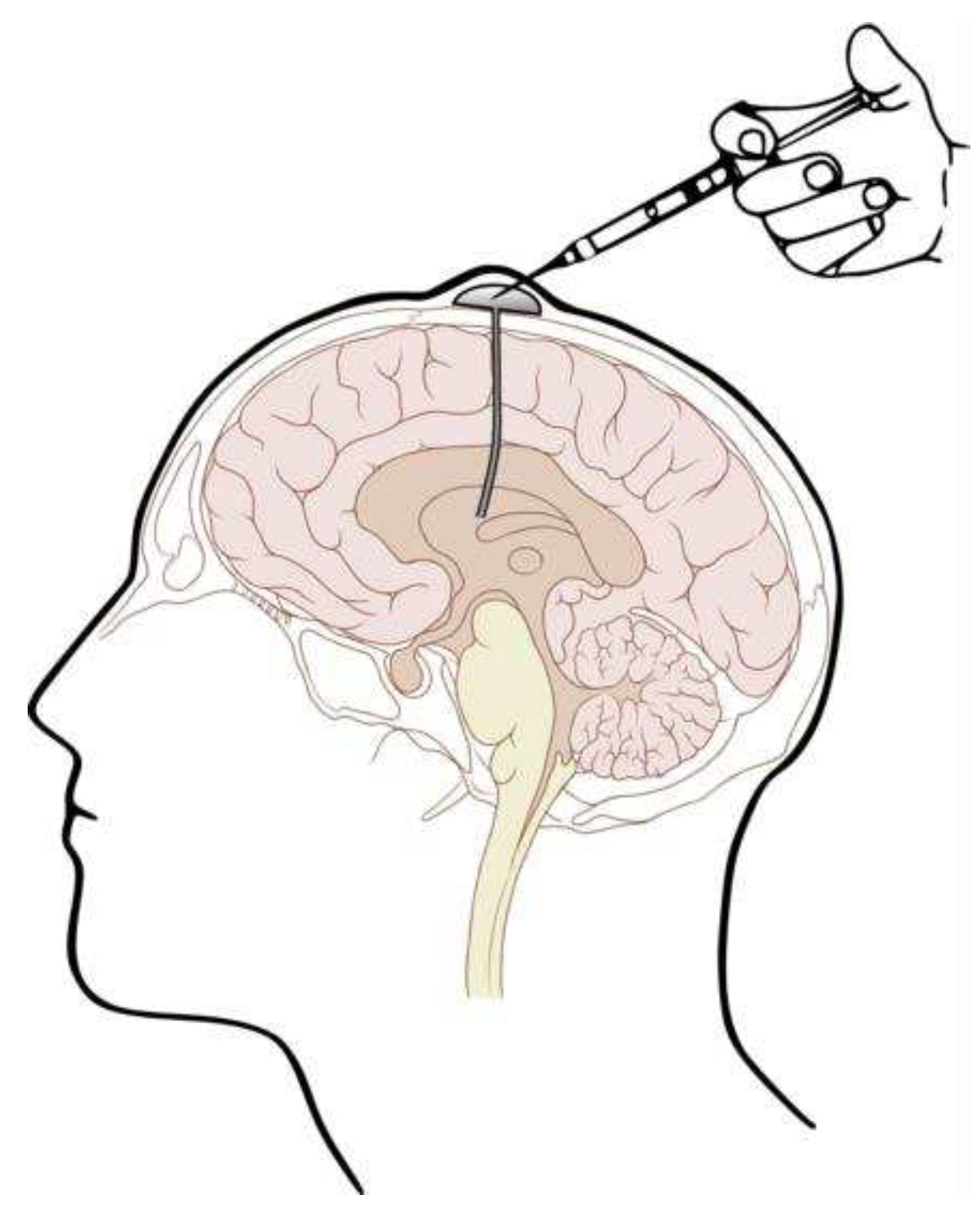
3.2. ICV Administration: Safety and Invasiveness
3.3. Scalability
3.4. A clinical Trial Worth Performing
3.5. Brain-Restricted mTOR Inhibition: A Breakthrough
4. Materials and Methods
4.1. Preparation of Everolimus Solution
4.2. Preparation of Everolimus Loaded Micelles
4.3. Everolimus Quantification
4.4. Cell Cultures
4.5. MTT Analysis
4.6. Cytotoxicity
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, L.S.; Vautier, M.; Allenbach, Y.; Zahr, N.; Benveniste, O.; Funck-Brentano, C.; Salem, J.-E. Sirolimus and MTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf. 2019, 42, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.J.; Su, Q. Mechanism of Action of the Immunosuppressant Rapamycin. Life Sci. 1995, 58, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous Sclerosis. Lancet Lond. Engl. 2008, 372, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shrikhande, G.; Xu, J.; McKay, R.M.; Burns, D.K.; Johnson, J.E.; Parada, L.F. Tsc1 Mutant Neural Stem/Progenitor Cells Exhibit Migration Deficits and Give Rise to Subependymal Lesions in the Lateral Ventricle. Genes Dev. 2011, 25, 1595–1600. [Google Scholar] [CrossRef]
- Franz, D.N.; Leonard, J.; Tudor, C.; Chuck, G.; Care, M.; Sethuraman, G.; Dinopoulos, A.; Thomas, G.; Crone, K.R. Rapamycin Causes Regression of Astrocytomas in Tuberous Sclerosis Complex. Ann. Neurol. 2006, 59, 490–498. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and Safety of Everolimus for Subependymal Giant Cell Astrocytomas Associated with Tuberous Sclerosis Complex (EXIST-1): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet Lond. Engl. 2013, 381, 125–132. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.A.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive Everolimus Therapy for Treatment-Resistant Focal-Onset Seizures Associated with Tuberous Sclerosis (EXIST-3): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Study. Lancet Lond. Engl. 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Chapman, N.M.; Chi, H. MTOR Signaling, Tregs and Immune Modulation. Immunotherapy 2014, 6, 1295–1311. [Google Scholar] [CrossRef]
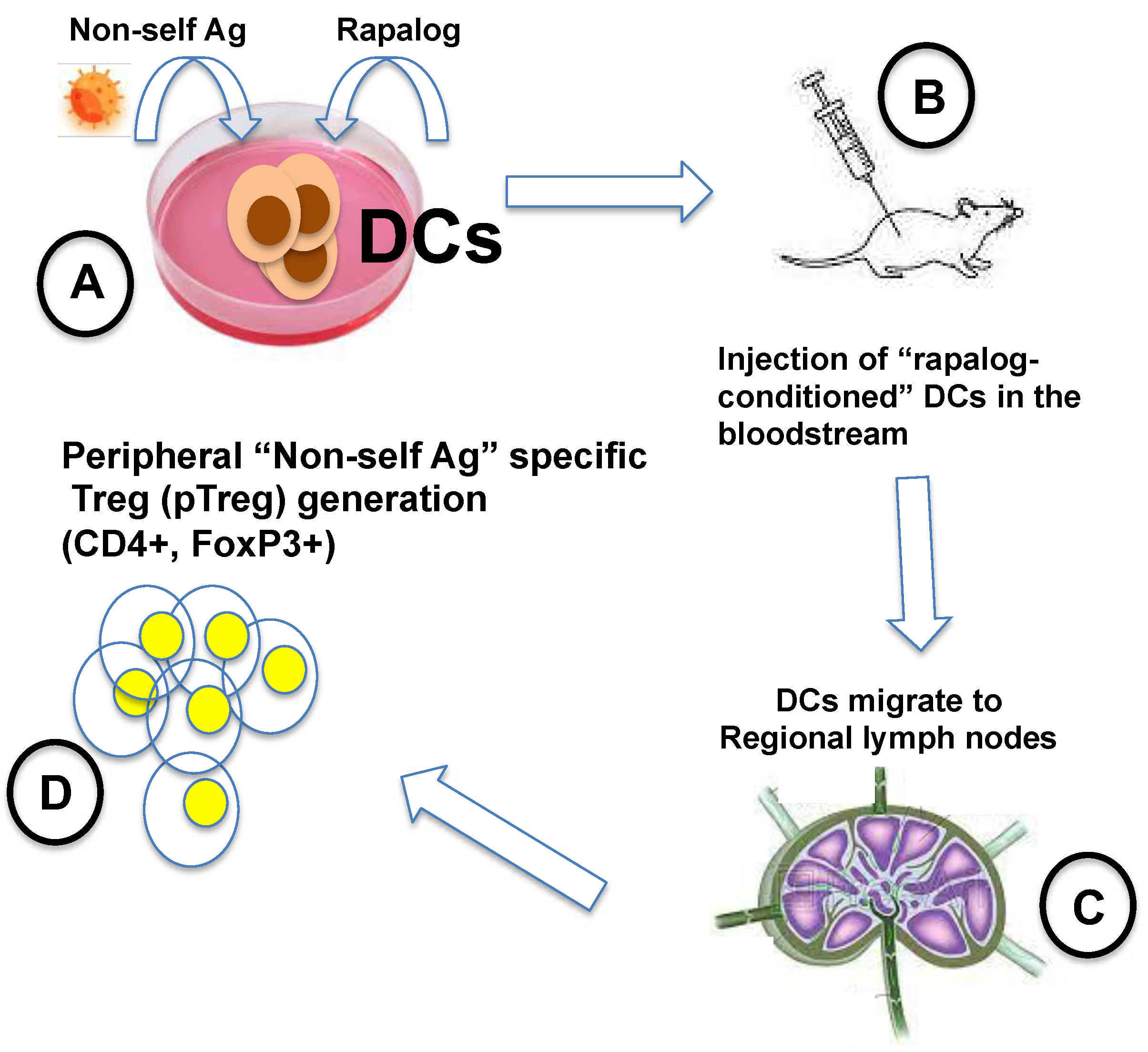
- Silk, K.M.; Leishman, A.J.; Nishimoto, K.P.; Reddy, A.; Fairchild, P.J. Rapamycin Conditioning of Dendritic Cells Differentiated from Human ES Cells Promotes a Tolerogenic Phenotype. J. Biomed. Biotechnol. 2012, 2012, 172420. [Google Scholar] [CrossRef]
- Stallone, G.; Infante, B.; Di Lorenzo, A.; Rascio, F.; Zaza, G.; Grandaliano, G. MTOR Inhibitors Effects on Regulatory T Cells and on Dendritic Cells. J. Transl. Med. 2016, 14, 152. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Raimondi, G.; Zahorchak, A.F.; Fischer, R.T.; Wang, Z.; Thomson, A.W. Rapamycin-Conditioned Dendritic Cells Are Poor Stimulators of Allogeneic CD4+ T Cells, but Enrich for Antigen-Specific Foxp3+ T Regulatory Cells and Promote Organ Transplant Tolerance. J. Immunol. Baltim. Md 1950 2007, 178, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Turnquist, H.R.; Taner, T.; Thomson, A.W. Use of Rapamycin in the Induction of Tolerogenic Dendritic Cells. Handb. Exp. Pharmacol. 2009, 215–232. [Google Scholar] [CrossRef]
- Macedo, C.; Turquist, H.; Metes, D.; Thomson, A.W. Immunoregulatory Properties of Rapamycin-Conditioned Monocyte-Derived Dendritic Cells and Their Role in Transplantation. Transplant. Res. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Ruffini, F.; Bellone, M.; Gagliani, N.; Battaglia, M.; Martino, G.; Furlan, R. Rapamycin Inhibits Relapsing Experimental Autoimmune Encephalomyelitis by Both Effector and Regulatory T Cells Modulation. J. Neuroimmunol. 2010, 220, 52–63. [Google Scholar] [CrossRef]
- Hou, H.; Miao, J.; Cao, R.; Han, M.; Sun, Y.; Liu, X.; Guo, L. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the MTOR-STAT3 Pathway. Neurochem. Res. 2017, 42, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, C.; Lisi, L.; Feinstein, D.L.; Navarra, P. MTOR Kinase, a Key Player in the Regulation of Glial Functions: Relevance for the Therapy of Multiple Sclerosis. Glia 2013, 61, 301–311. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhang, B.; Liu, W.; Sun, M.-J.; Zhang, Y.-L.; Liu, H.; Wang, M.-X. Rapamycin Alleviates the Symptoms of Multiple Sclerosis in Experimental Autoimmune Encephalomyelitis (EAE) Through Mediating the TAM-TLRs-SOCS Pathway. Front. Neurol. 2020, 11, 590884. [Google Scholar] [CrossRef]
- Li, Z.; Nie, L.; Chen, L.; Sun, Y.; Li, G. Rapamycin Relieves Inflammation of Experimental Autoimmune Encephalomyelitis by Altering the Balance of Treg/Th17 in a Mouse Model. Neurosci. Lett. 2019, 705, 39–45. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Alexaki, A.; Brinia, M.-E.; Anagnostouli, M.; Stefanis, L.; Stathopoulos, P. The MTOR Signaling Pathway in Multiple Sclerosis; from Animal Models to Human Data. Int. J. Mol. Sci. 2022, 23, 8077. [Google Scholar] [CrossRef]
- Bagherpour, B.; Salehi, M.; Jafari, R.; Bagheri, A.; Kiani-Esfahani, A.; Edalati, M.; Kardi, M.T.; Shaygannejad, V. Promising Effect of Rapamycin on Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 26, 40–45. [Google Scholar] [CrossRef]
- Monti, P.; Scirpoli, M.; Maffi, P.; Piemonti, L.; Secchi, A.; Bonifacio, E.; Roncarolo, M.-G.; Battaglia, M. Rapamycin Monotherapy in Patients with Type 1 Diabetes Modifies CD4+CD25+FOXP3+ Regulatory T-Cells. Diabetes 2008, 57, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Abeta and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular Interplay between Mammalian Target of Rapamycin (MTOR), Amyloid-Beta, and Tau: Effects on Cognitive Impairments. J. Biol. Chem. 2010, 285, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Haage, V.; De Jager, P.L. Neuroimmune Contributions to Alzheimer’s Disease: A Focus on Human Data. Mol. Psychiatry 2022, 27, 3164–3181. [Google Scholar] [CrossRef]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of MTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-Beta Levels in a Mouse Model of Alzheimer’s Disease. PloS One 2010, 5, e9979. [Google Scholar] [CrossRef]
- Lin, A.-L.; Zheng, W.; Halloran, J.J.; Burbank, R.R.; Hussong, S.A.; Hart, M.J.; Javors, M.; Shih, Y.-Y.I.; Muir, E.; Fonseca, R.S.; et al. Chronic Rapamycin Restores Brain Vascular Integrity and Function Through NO Synthase Activation and Improves Memory in Symptomatic Mice Modeling Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2013, 33, 1412–1421. [Google Scholar] [CrossRef]
- Lin, A.-L.; Jahrling, J.B.; Zhang, W.; DeRosa, N.; Bakshi, V.; Romero, P.; Galvan, V.; Richardson, A. Rapamycin Rescues Vascular, Metabolic and Learning Deficits in Apolipoprotein E4 Transgenic Mice with Pre-Symptomatic Alzheimer’s Disease. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 217–226. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin Fed Late in Life Extends Lifespan in Genetically Heterogeneous Mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Dolcetta, D.; Dominici, R. The Local Mammalian Target of Rapamycin (MTOR) Modulation: A Promising Strategy to Counteract Neurodegeneration. Neural Regen. Res. 2019, 14, 1711–1712. [Google Scholar] [CrossRef]
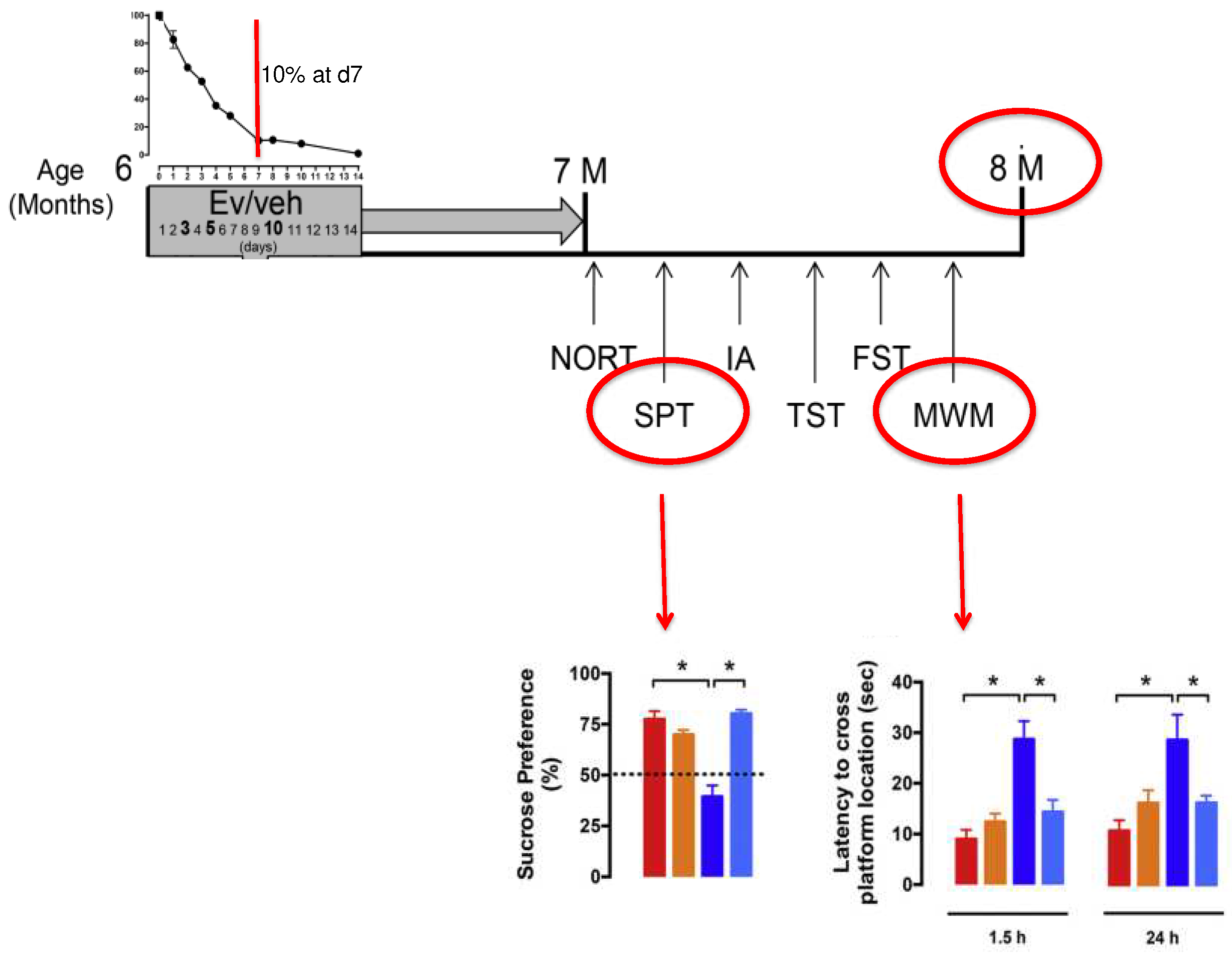
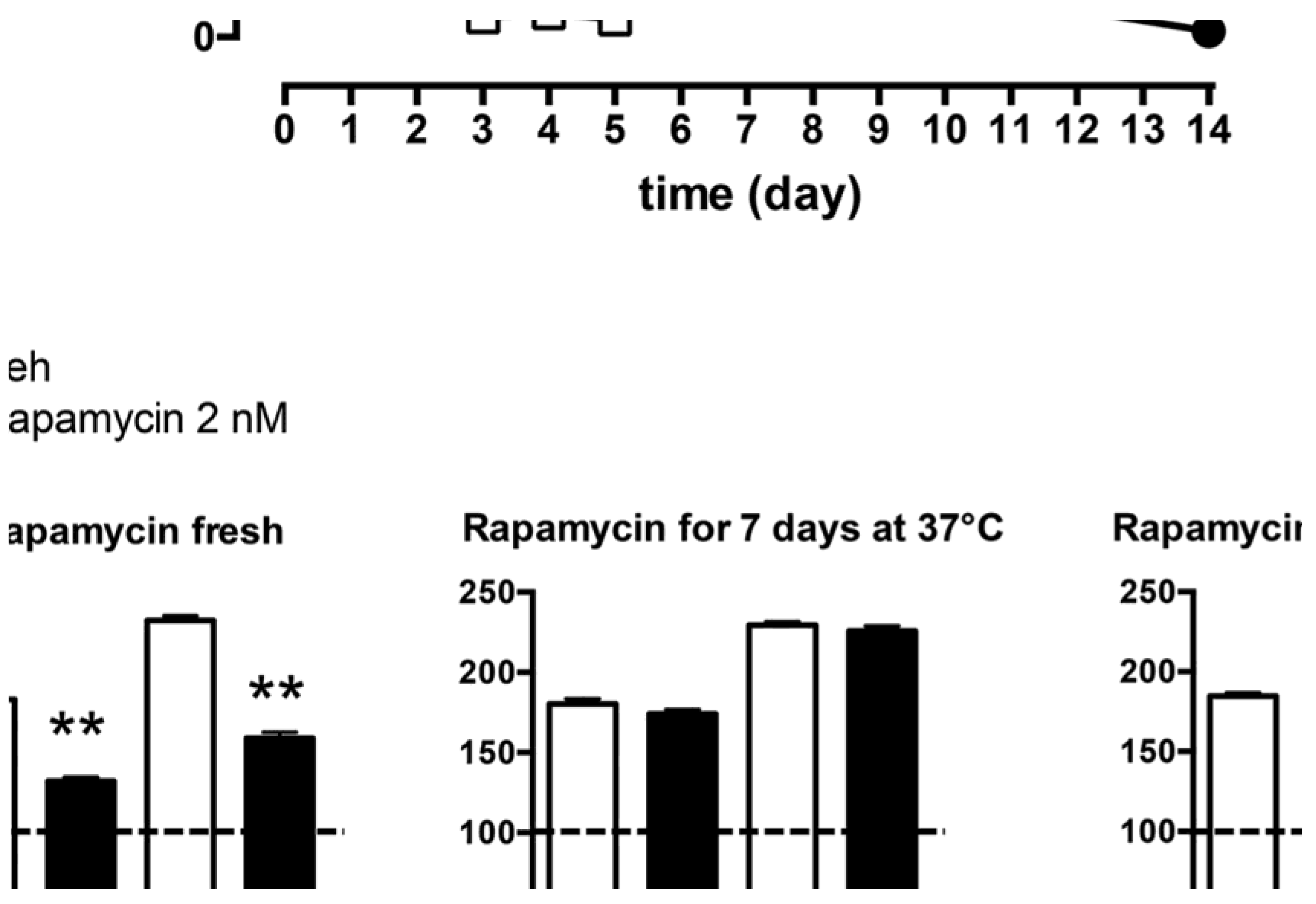
- Cassano, T.; Magini, A.; Giovagnoli, S.; Polchi, A.; Calcagnini, S.; Pace, L.; Lavecchia, M.A.; Scuderi, C.; Bronzuoli, M.R.; Ruggeri, L.; et al. Early Intrathecal Infusion of Everolimus Restores Cognitive Function and Mood in a Murine Model of Alzheimer’s Disease. Exp. Neurol. 2019, 311, 88–105. [Google Scholar] [CrossRef]
- Atkinson, A.J. Intracerebroventricular Drug Administration. Transl. Clin. Pharmacol. 2017, 25, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Polymeric Micellar Systems-A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, D.; Han, S.; Shilyansky, C.; Zhou, Y.; Li, W.; Kwiatkowski, D.J.; Ramesh, V.; Silva, A.J. Reversal of Learning Deficits in a Tsc2+/- Mouse Model of Tuberous Sclerosis. Nat. Med. 2008, 14, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.G.; Tsai, V.W.W.; Ruitenberg, M.J.; Hassanpour, M.; Li, H.; Hart, P.H.; Breit, S.N.; Sawchenko, P.E.; Brown, D.A. Immune Cell Trafficking from the Brain Maintains CNS Immune Tolerance. J. Clin. Invest. 2014, 124, 1228–1241. [Google Scholar] [CrossRef]
- Yang, H.; Park, S.-Y.; Baek, H.; Lee, C.; Chung, G.; Liu, X.; Lee, J.H.; Kim, B.; Kwon, M.; Choi, H.; et al. Adoptive Therapy with Amyloid-β Specific Regulatory T Cells Alleviates Alzheimer’s Disease. Theranostics 2022, 12, 7668–7680. [Google Scholar] [CrossRef] [PubMed]
- Faridar, A.; Thome, A.D.; Zhao, W.; Thonhoff, J.R.; Beers, D.R.; Pascual, B.; Masdeu, J.C.; Appel, S.H. Restoring Regulatory T-Cell Dysfunction in Alzheimer’s Disease through Ex Vivo Expansion. Brain Commun. 2020, 2, fcaa112. [Google Scholar] [CrossRef]
- Weaver, D.F. Alzheimer’s Disease as an Innate Autoimmune Disease (AD2 ): A New Molecular Paradigm. Alzheimers Dement. J. Alzheimers Assoc. 2022. [Google Scholar] [CrossRef]
- Liston, A.; Dooley, J.; Yshii, L. Brain-Resident Regulatory T Cells and Their Role in Health and Disease. Immunol. Lett. 2022, 248, 26–30. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Wang, C.; Han, T.; Liu, H.; Sun, L.; Hong, J.; Hashimoto, M.; Wei, J. The Reciprocal Interactions between Microglia and T Cells in Parkinson’s Disease: A Double-Edged Sword. J. Neuroinflammation 2023, 20, 33. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Pinheiro, L.; Brem, H. Delivery of Local Therapeutics to the Brain: Working toward Advancing Treatment for Malignant Gliomas. Ther. Deliv. 2015, 6, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Peyrl, A.; Chocholous, M.; Azizi, A.A.; Czech, T.; Dorfer, C.; Mitteregger, D.; Gojo, J.; Minichmayr, E.; Slavc, I. Safety of Ommaya Reservoirs in Children with Brain Tumors: A 20-Year Experience with 5472 Intraventricular Drug Administrations in 98 Patients. J. Neurooncol. 2014, 120, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Giordan, E.; Palandri, G.; Lanzino, G.; Murad, M.H.; Elder, B.D. Outcomes and Complications of Different Surgical Treatments for Idiopathic Normal Pressure Hydrocephalus: A Systematic Review and Meta-Analysis. J. Neurosurg. 2018, 1–13. [Google Scholar] [CrossRef]
- Ponjoan, A.; Garre-Olmo, J.; Blanch, J.; Fages, E.; Alves-Cabratosa, L.; Martí-Lluch, R.; Comas-Cufí, M.; Parramon, D.; Garcia-Gil, M.; Ramos, R. Epidemiology of Dementia: Prevalence and Incidence Estimates Using Validated Electronic Health Records from Primary Care. Clin. Epidemiol. 2019, 11, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, Q.; Luo, X.; Lou, K.; Weiss, W.A.; Shokat, K.M. Brain-Restricted MTOR Inhibition with Binary Pharmacology. Nature 2022, 609, 822–828. [Google Scholar] [CrossRef]
- Kocak, M.; Ezazi Erdi, S.; Jorba, G.; Maestro, I.; Farrés, J.; Kirkin, V.; Martinez, A.; Pless, O. Targeting Autophagy in Disease: Established and New Strategies. Autophagy 2022, 18, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Alruwais, N.M.; Rusted, J.M.; Tabet, N.; Dowell, N.G. Evidence of Emerging BBB Changes in Mid-Age Apolipoprotein E Epsilon-4 Carriers. Brain Behav. 2022, 12, e2806. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Galstyan, A.; Grodzinski, Z.B.; Shatalova, E.S.; Wagner, S.; Israel, L.L.; Ding, H.; Black, K.L.; Ljubimova, J.Y.; Holler, E. Single- and Multi-Arm Gadolinium MRI Contrast Agents for Targeted Imaging of Glioblastoma. Int. J. Nanomedicine 2020, 15, 3057–3070. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




