Submitted:
01 March 2023
Posted:
01 March 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Animal Experiments
Blood Sampling
Tissue Collection
Analysis of Phosphatidylcholine Concentration in the Liver Tissues
Western Blotting
Detection of Carbonyl Modified Proteins (2D-Oxyblot Analysis)
Two-Dimensional Differential in-Gel Electrophoretic (2D-DIGE) Analysis
Histological and Immunofluorescence Histochemical Analyses
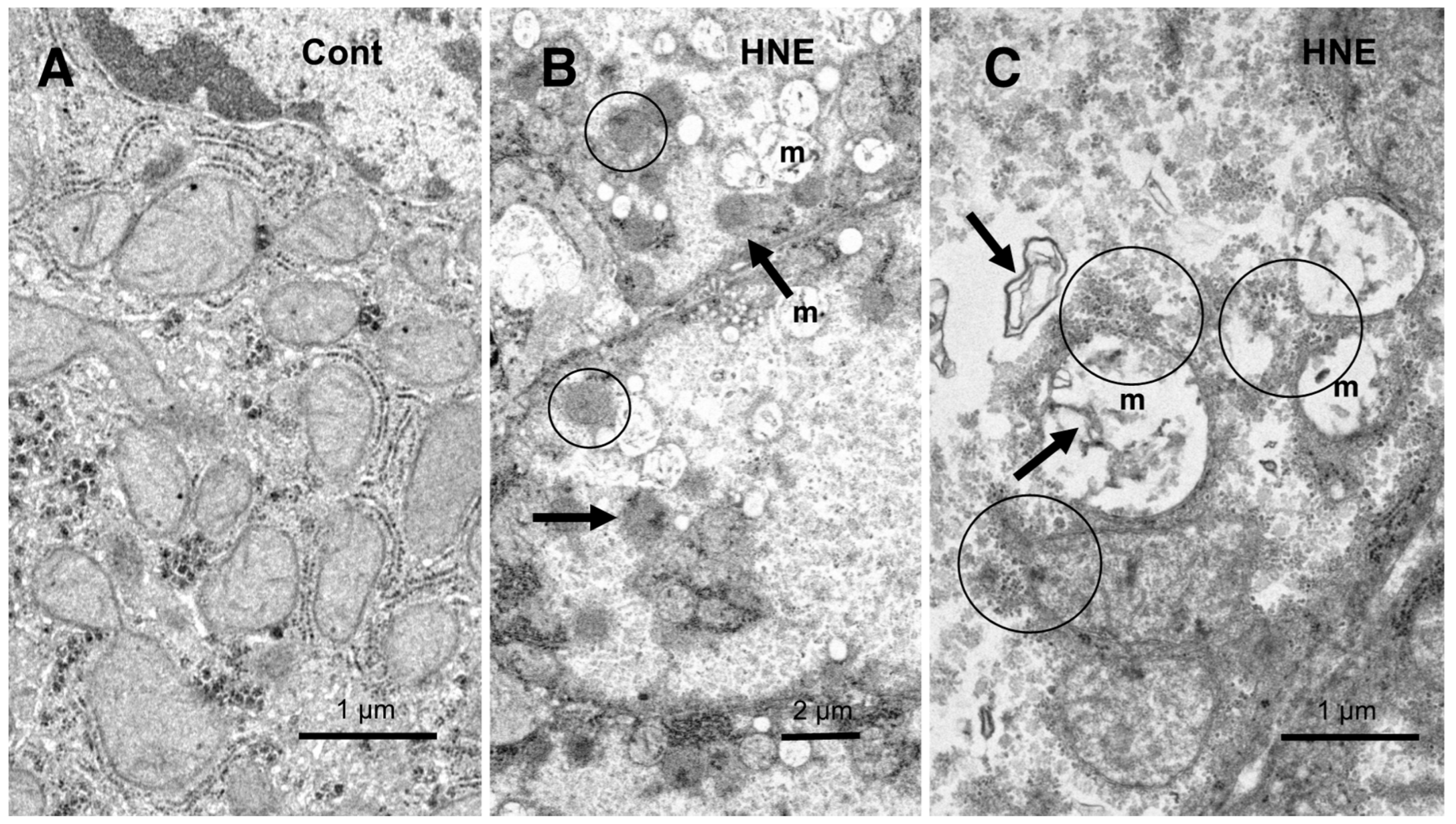
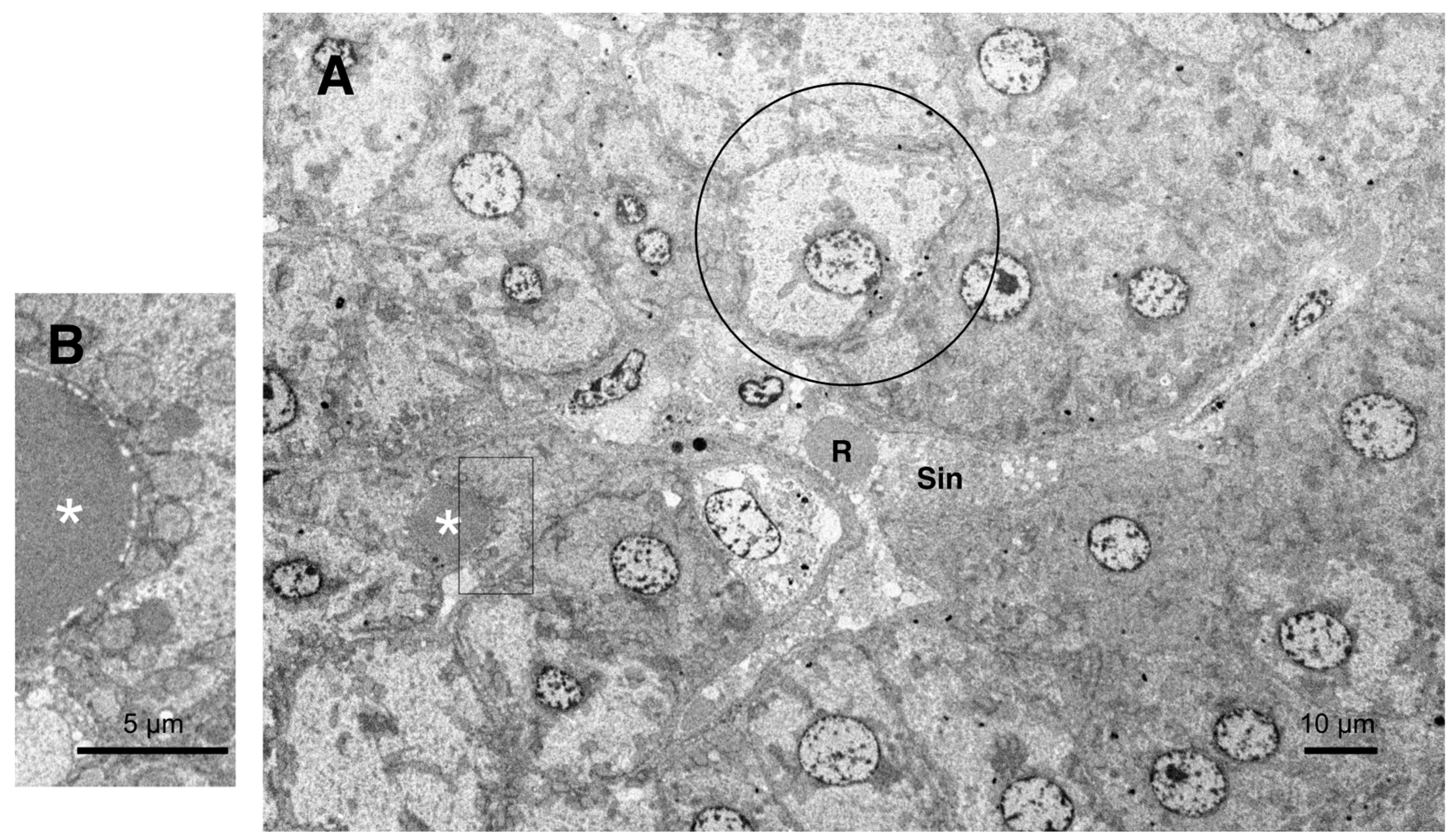
Ultrastructural Analyses
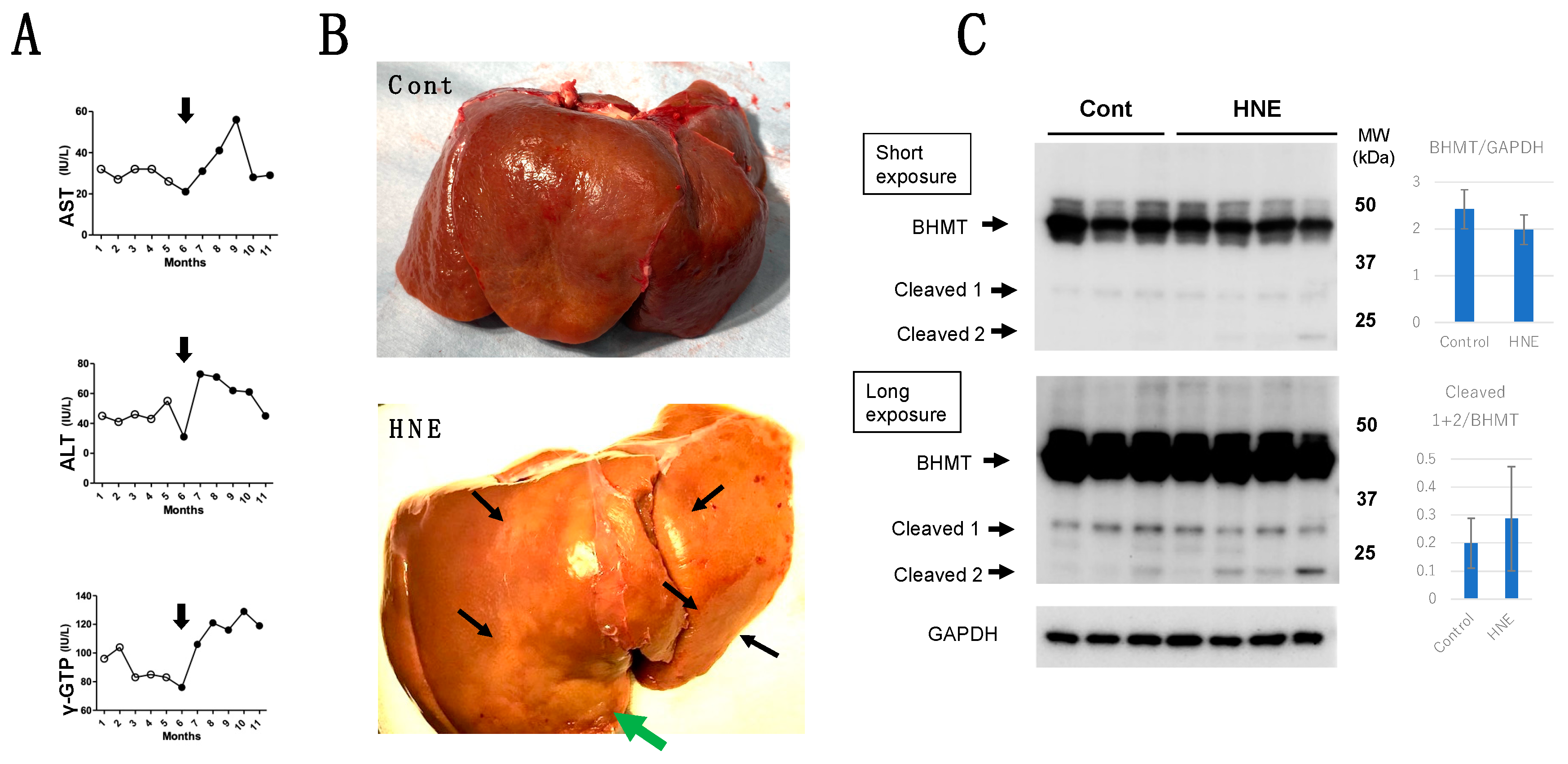
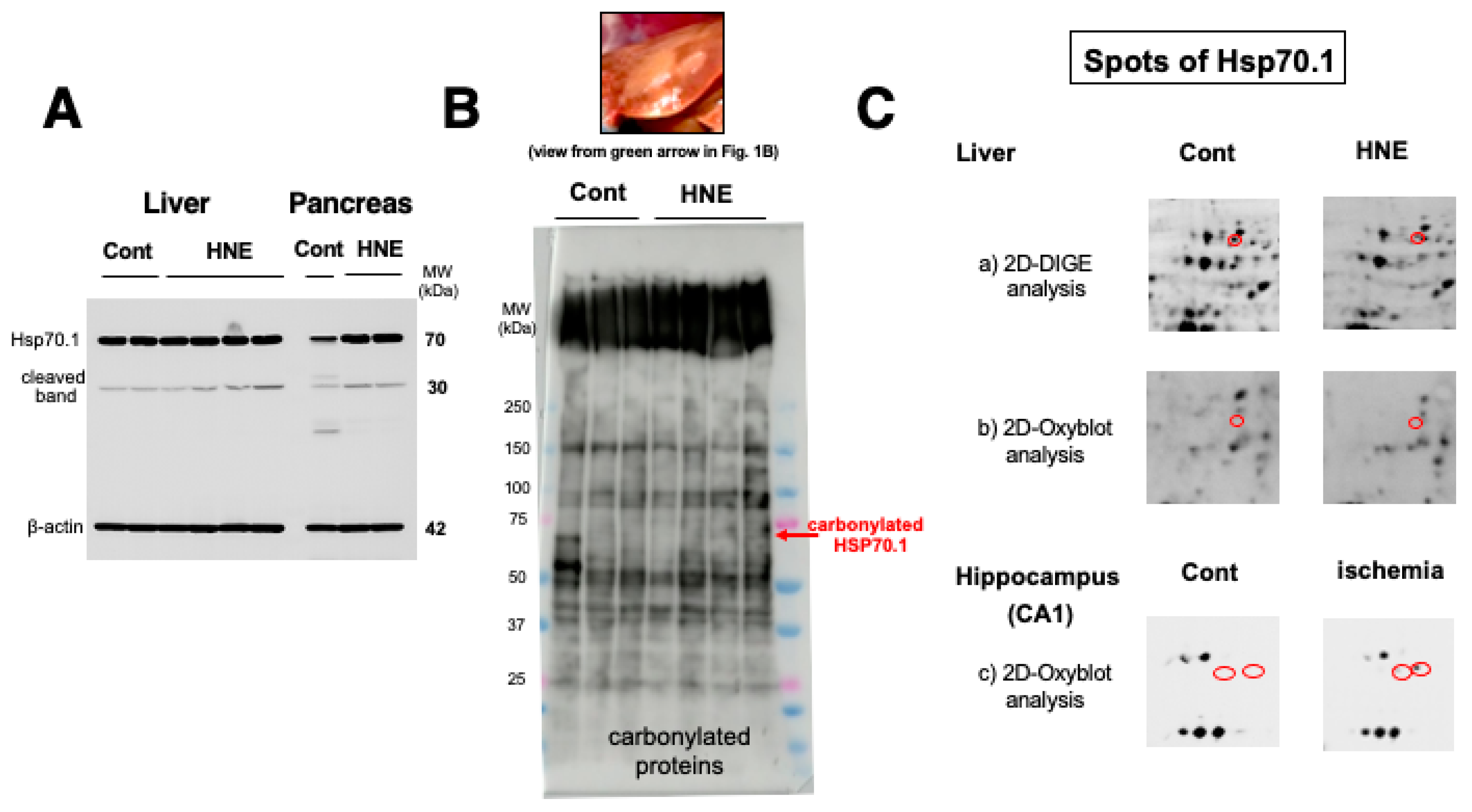
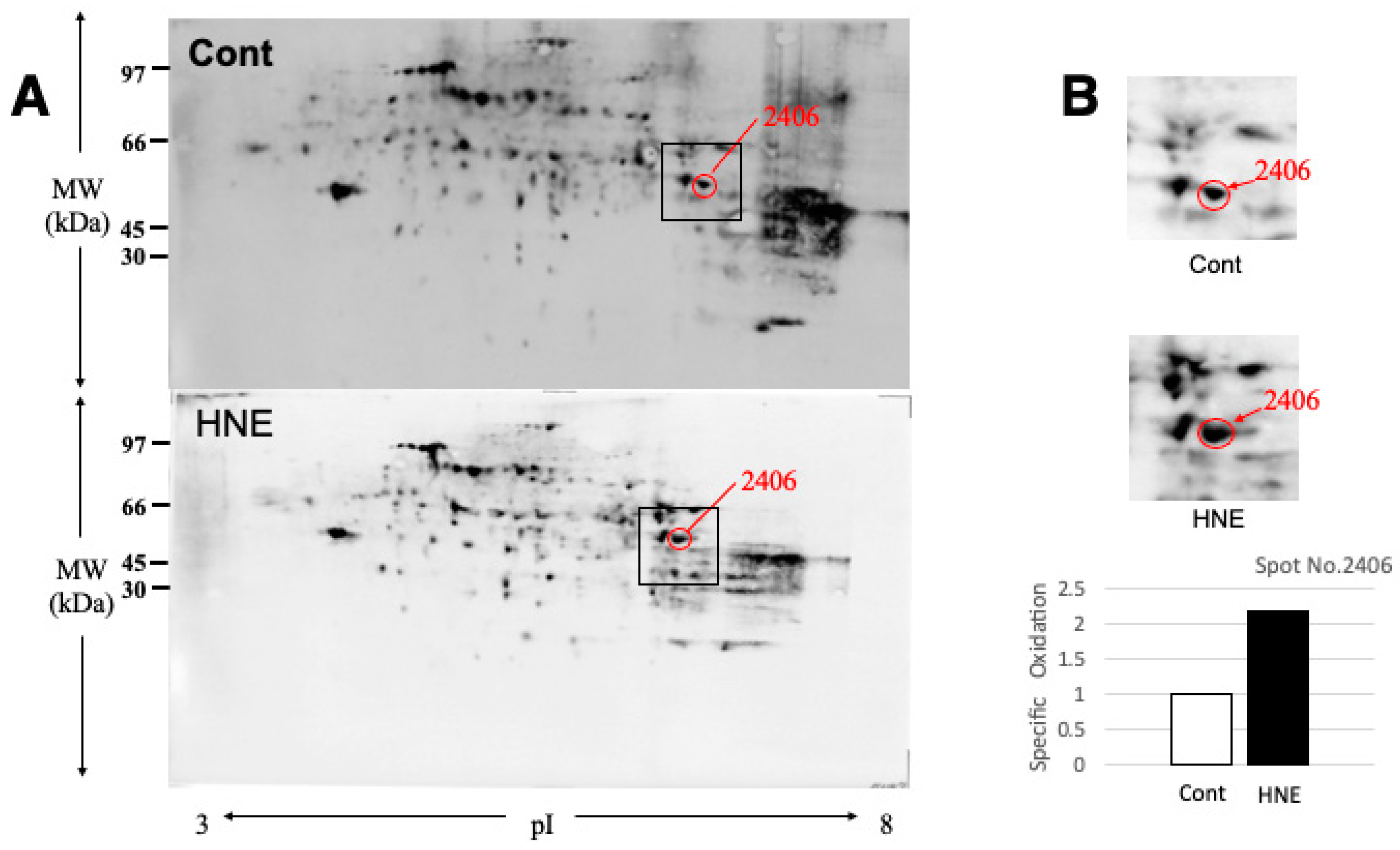
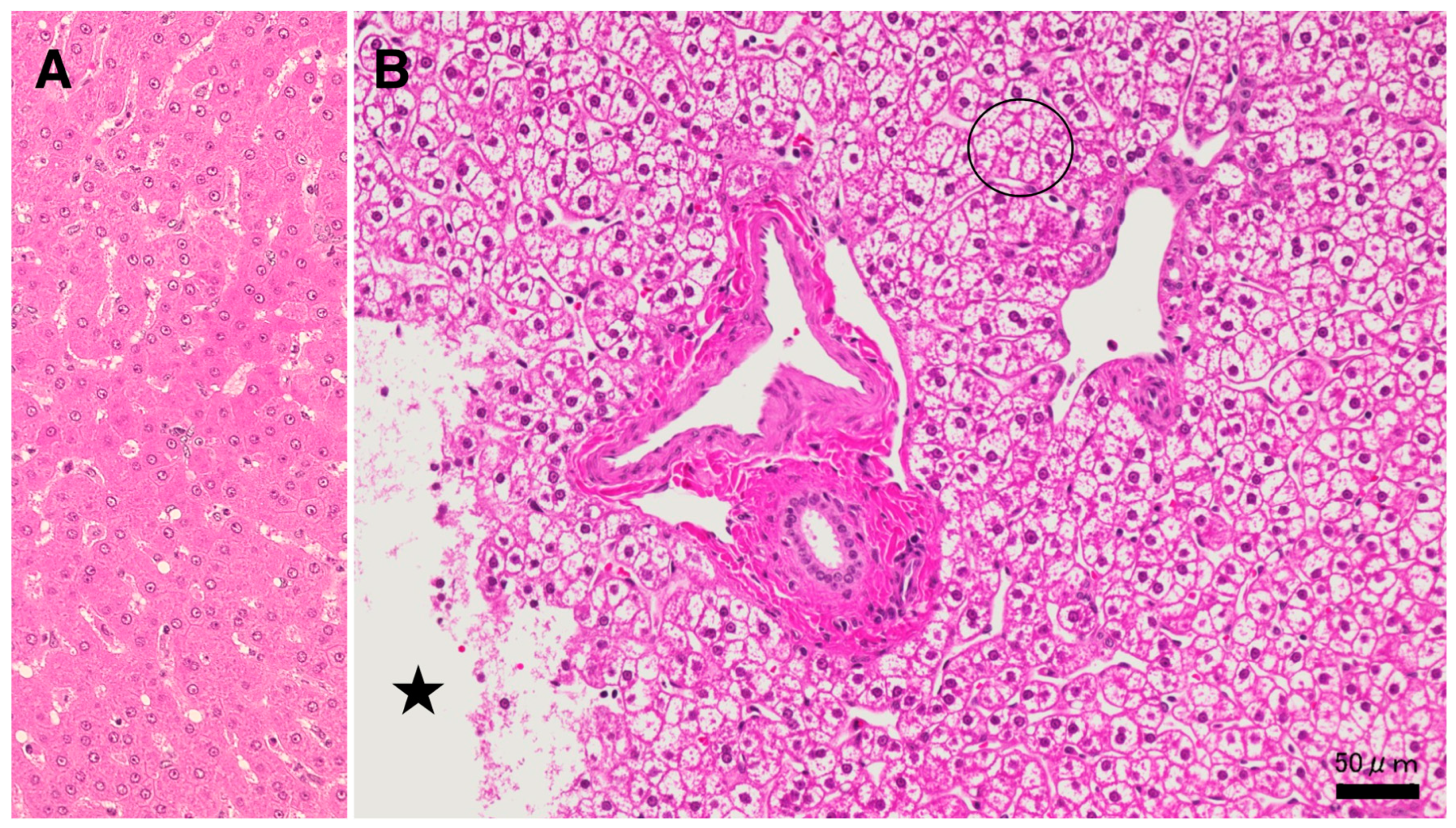
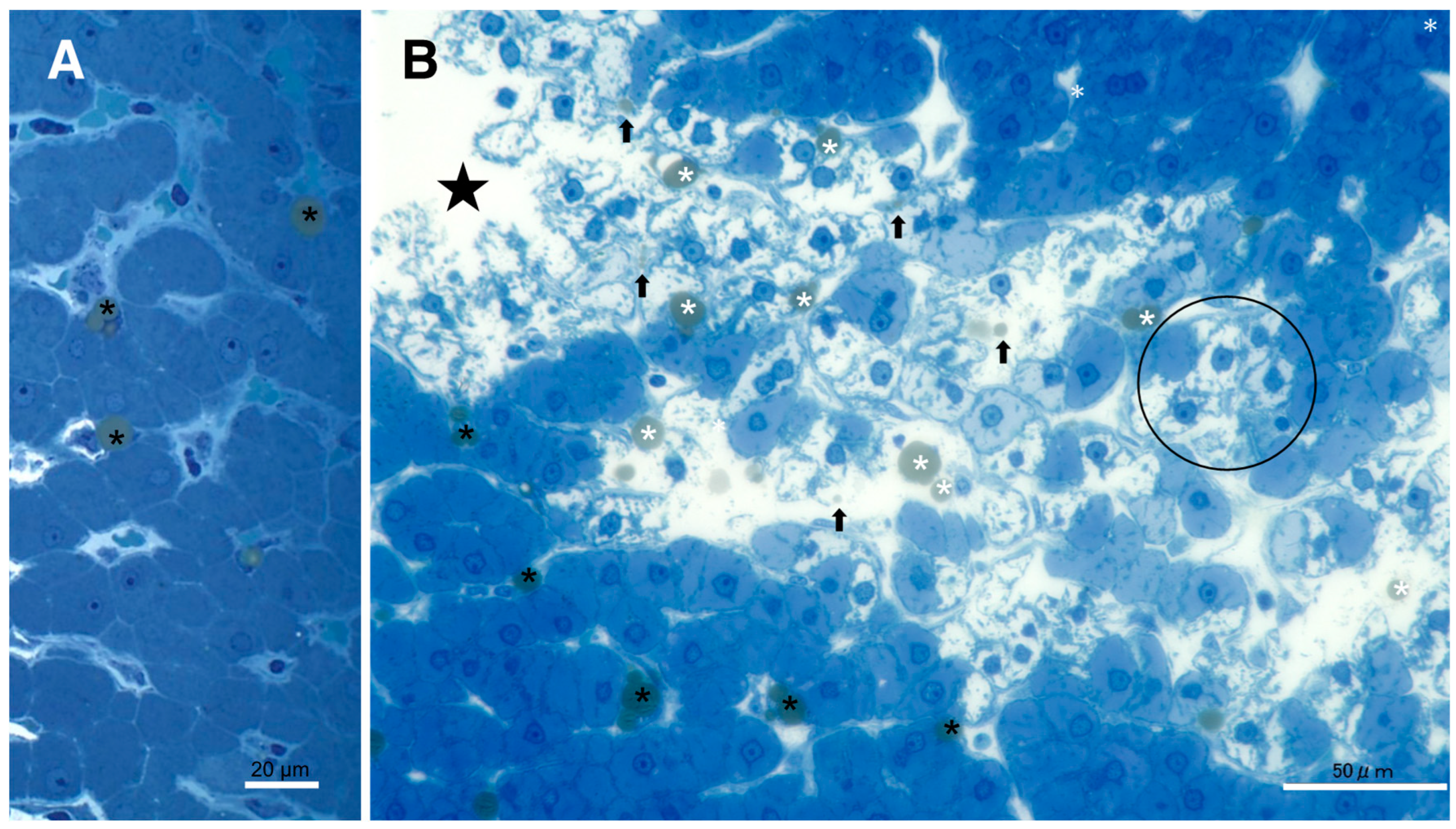
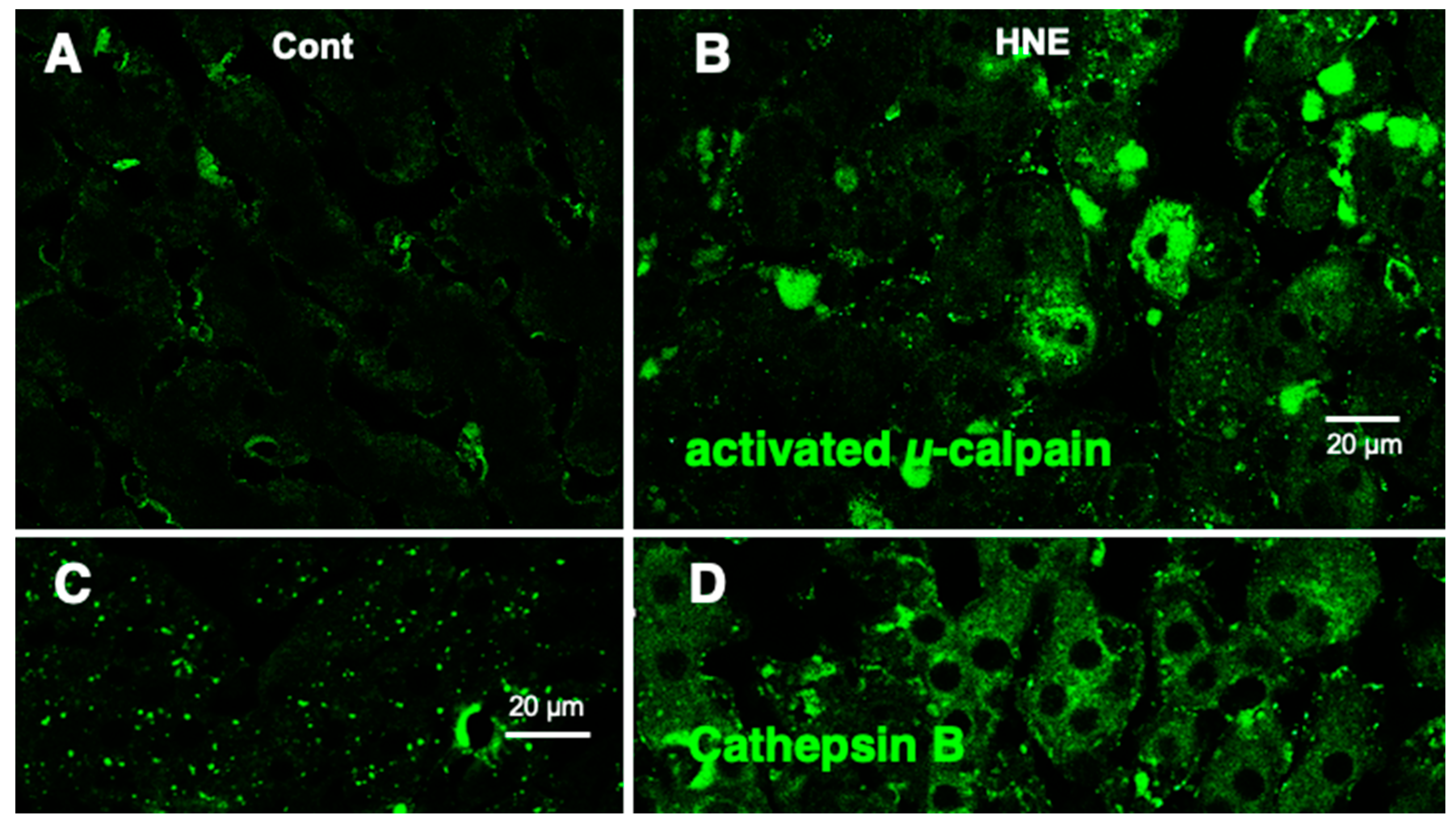
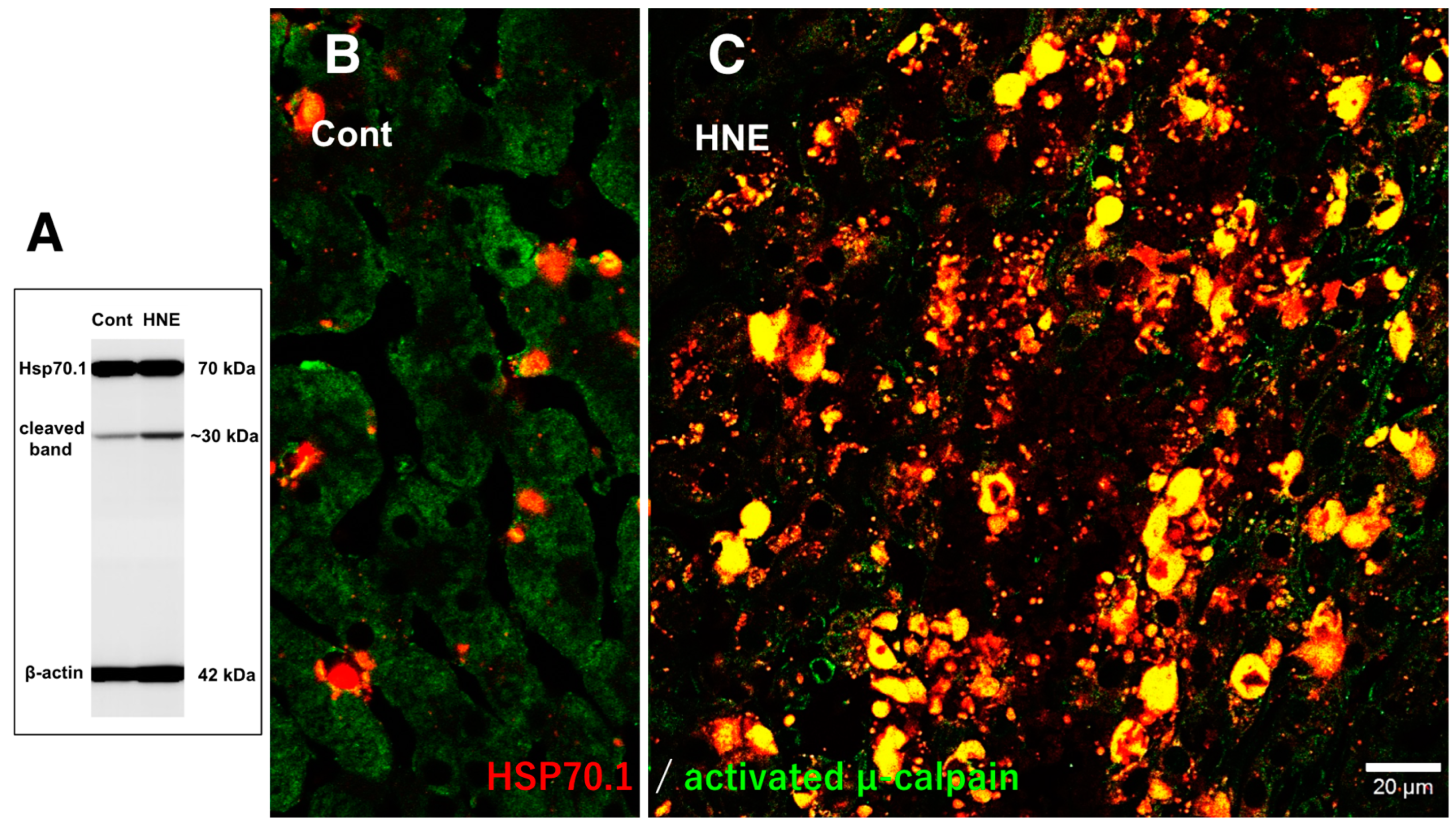
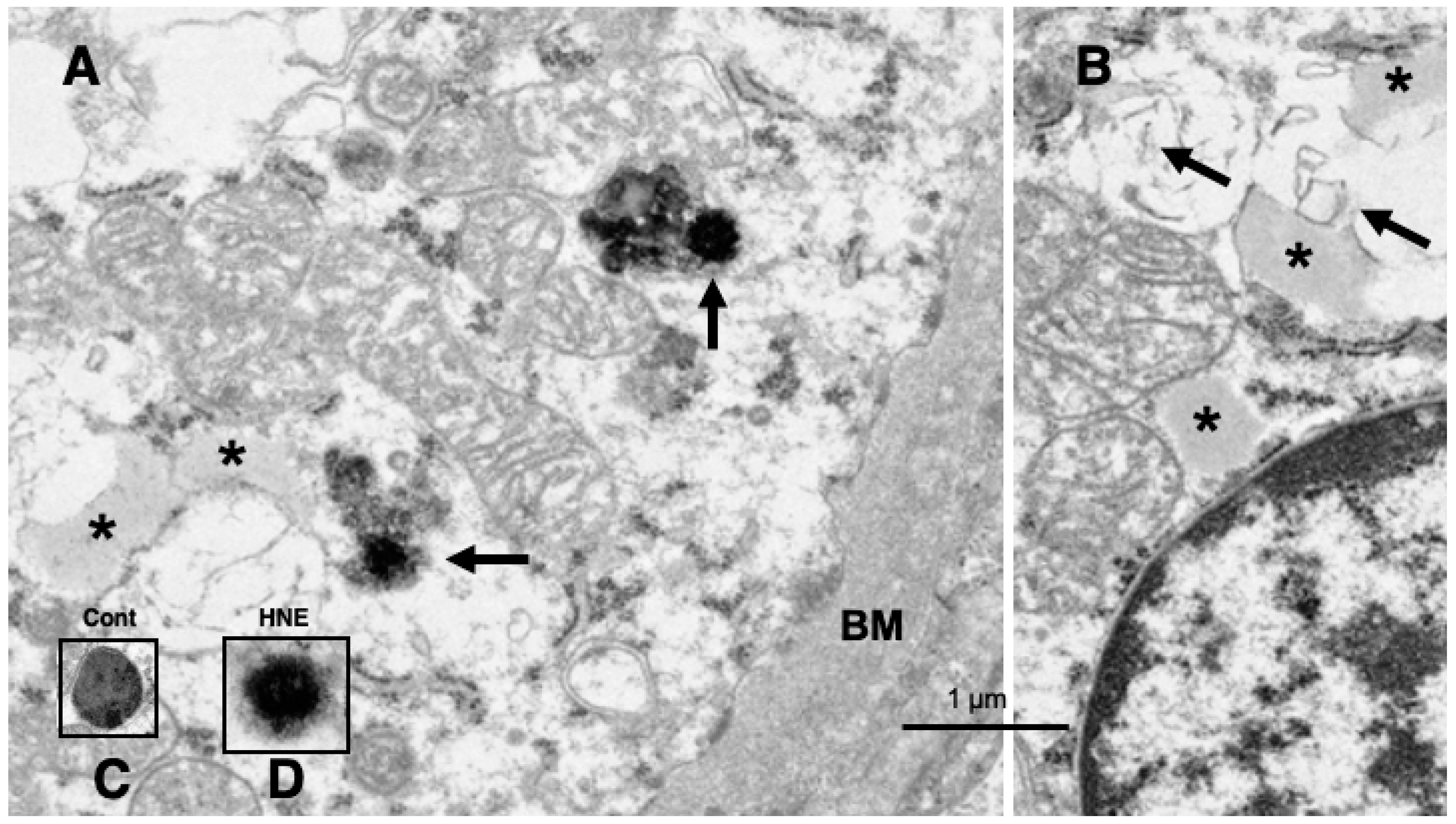
Results
Discussion
Hydroxynonenal-Induced Hsp70.1 Deficiency Causes Lysosomal Membrane Disintegrity and Cell Degeneration
BHMT Carbonylation and Cleavage Cause Phosphatidylcholine Decrease and Hepatic Steatosis
Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ALDH2 | aldehyde dehydrogenase 2 |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| γ-GTP | γ-glutamyl transferase |
| BHMT | betaine-homocysteine S-methyltransferase |
| DNP | 2,4-dinitrophenylhydrazone |
| DNPH | 2,4-dinitrophenylhydrazine |
| Hsp70.1 | heat-shock protein 70.1 |
| MALDI-TOF/TOF MS | matrix-assisted laser desorption ionization time-of-flight tandem mass spectrometry |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| PEMT pathway | phosphatidyl-ethanolamine methyltransferase pathway |
| PUFA | polyunsaturated fatty acid |
| 2D-DIGE | two-dimensional differential in-gel electrophoretic |
| VLDL | very low-density lipoproteins |
References
- Friedman, J.M. Obesity in the new millennium. Nature 2000, 404, 632–634. [Google Scholar] [CrossRef]
- Diehl, A.M. Fatty liver, hypertension, and the metabolic syndrome. Gut 2004, 53, 923–924. [Google Scholar] [CrossRef]
- Wieckowska, A.; Feldstein, A.E. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr. Opin. Pediatr. 2005, 17, 636–641. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. New Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Brancati, F.L.; Diehl, AM. Nonalcoholic fatty liver disease. 2002, 122, 1649–1657. [Google Scholar] [CrossRef]
- Brunt, E.M.; Tiniakos, DG. Pathology of steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 691–707. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; Rizzetto, M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Harrison, S.A.; Neuschwander-Tetri, B.A. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin. Liver Dis. 2004, 8, 861–879. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younoss, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Seike, T.; Boontem, P.; Yanagi, M.; Li, S.; Kido, H.; Yamamiya, D.; Nakagawa, H.; Okada, H.; Yamashita, T.; Harada, K.; Kikuchi, M.; Shiraishi, Y.; Ozaki, N.; Kaneko, S.; Yamashima, T.; Mizukoshi, E. Hydroxynonenal causes hepatocyte death by disrupting lysosomal integrity in non-alcoholic steatohepatitis. Cell. Mol. Gastro. Hepatol. 2022, 14, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009, 44, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Ota, T.; Mizukoshi, E.; Nakamura, H.; Yamamoto, Y.; Kikuchi, M.; Yamashita, T.; Kaneko, S. Intake of ω-6 polyunsaturated fatty acid-rich vegetable oils and risk of lifestyle diseases. Adv. Nutr. 2020, 11, 1489–1509. [Google Scholar] [CrossRef] [PubMed]
- Boontem, P.; Yamashima, T. Hydroxynonenal causes Langerhans cell degeneration in the pancreas of Japanese macaque monkeys. PLoS ONE 2021, 16(11), e0245702. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006, 8(2), 203. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, P.A.; Staniszewska, A.D.; Li, W.; Omidvar, N.; Kedjouar, B.; Turkson, J.; Poli, V.; Flavell, R.A. , Clarkson, R.W.; Watson, C.J. Stat3 controls lysosomal-mediated cell death in vivo. Nat. Cell Biol. 2011, 13, 303–309. [Google Scholar] [CrossRef]
- Sargeant, T.J.; Lloyd-Lewis, B.; Resemann, H.K.; Ramos-Montoya, A.; Skepper, J.; Watson, C.J. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nature Cell Biol. 2014, 16, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Kohda, Y.; Tsuchiya, K.; Ueno, T.; Yamashita, J.; Yoshioka, T.; Kominamin, E. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: A novel strategy for neuroprotection based on ’calpain-cathepsin hypothesis’. Eur. J. Neurosci. 1998, 10, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Oikawa, S. The role of lysosomal rupture in neuronal death. Prog. Neurobiol. 2009, 89, 343–358. [Google Scholar] [CrossRef]
- Sahara, S.; Yamashima, T. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem. Biophys. Res. Commun. 2010, 393, 806–811. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; Horvath, I.; Mestril, R.; Watt, M.J.; Hooper, P.L.; Kingwell, B.A.; Vigh, L.; Hevener, A.; Febbraio, M.A. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA, 2008; 105, 1739–1744. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Bruce, C.R.; Drew, B.G.; Tory, K.; Kolonics, A.; Estevez, E.; Chung, J.; Watson, N.; Gardner, T.; Lee-Young, R.S.; Connor, T.; Watt, M.J.; Carpenter, K.; Hargreaves, M.; McGee, S.L.; Hevener, A.L.; Febbraio, M.A. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014, 63, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. Lipid Res. J. 2008, 49, 1187–1194. [Google Scholar] [CrossRef]
- 24. Suzuki, M.; Shinohara, Y.; Ohsaki, Y.; Fujimoto, T. Lipid droplets: Size matters. J. Electron Microsc. (Tokyo) 2011. 60 Supplement 1:S101–16. [CrossRef]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; Farese, Jr. R.V; Walther, T.C. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, LA. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef]
- Noga, A.A.; Zhao, Y.; Vance, D.E. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J. Biol. Chem. 2002, 277, 42358–42365. [Google Scholar] [CrossRef]
- Pajares, M.A.; Pérez-Sala, D. Betaine-homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 2006, 63, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, S.S.; Castro, C.C.; Koutmos, M.; Garrow, T.A. Betaine homocysteine S-methyltransferase-2 is an S-methylmethionine-homocysteine methyltransferase. J. Biol. Chem. 2008, 283, 8939–8945. [Google Scholar] [CrossRef]
- Teng, Y.W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Shinohara, M.; Vance, D.; Than, T. A.; Ookhtens, M.; Chan, C.; Kaplowitz, N. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcohol. Clin Exp. Res. 2008, 32, 1049–1058. [Google Scholar] [CrossRef]
- Chaudhuri, A.R.; de Waal, E.M.; Pierce, A.; Van Remmen, H.; Ward, W.F.; Richardson, A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech. Ageing Dev. 2006, 127, 849–861. [Google Scholar] [CrossRef]
- Doorn, J.A.; Petersen, D.R. ; Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002, 15, 1445–1450. [Google Scholar] [CrossRef]
- Uchida, K. Histidine and lysine as targets of oxidative modification. Amino Acids 2003, 25, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S., Yamada, T., Minohata, T., Kobayashi, H., Furukawa, A., Tada-Oikawa, S., Hiraku, Y.; Murata, M.; Kikuchi, M.; Yamashima, T. Proteomic identification of carbonylated proteins in the monkey hippocampus after ischemia-reperfusion. Free Radic. Biol. Med. 2009. 46, 1472–1477. [CrossRef] [PubMed]
- Newton, B.W.; Russell, W.K.; Russell, D.H.; Ramaiah, S.; Jayaraman, A. Liver proteome analysis in a rodent model of alcoholic steatosis. J. Proteome Res. 2009, 8, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.T.; McGleenon, B.M.; Brennan, S.; McColl, D.; McIlroy, S. Passmore, A.P. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. Q. J. Med. 2001, 94, 485–490. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Morita, S.; Tsuji, T.; Terada, T. Protocols for enzymatic fluorometric assays to quantify phospholipid classes. Int. J. Mol. Sci. 2020, 21, 1032. [Google Scholar] [CrossRef]
- Nakamura, A.; Goto, S. Analysis of protein carbonyls with 2,4-dinitrophenyl hydrazine and its antibodies by immunoblot in two-dimensional gel electrophoresis. J. Biochem. 1996, 119, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Nabeshi, H.; Oikawa, S.; Inoue, S.; Nishino, K.; Kawanishi, S. Proteomic analysis for protein carbonyl as an indicator of oxidative damage in senescence-accelerated mice. Free Radic. Res. 2006, 40, 1173–1181. [Google Scholar] [CrossRef]
- Mori, Y.; Oikawa, S.; Kurimoto, S.; Kitamura, Y.; Tada-Oikawa, S.; Kobayashi, H.; Yamashima, T.; Murata, M. Proteomic analysis of the monkey hippocampus for elucidating ischemic resistance. J. Clin. Biochem. Nutr. 2020, 67, 167–173. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J, N. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Song, K.X.; Qu, S. Nonalcoholic Fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective. Oxid. Med. Cell Longev. 2014, 2014, 637027. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom. Rev. 2005, 24, 55–99. [Google Scholar] [CrossRef] [PubMed]
- Heydari, A.R.; Takahashi, R.; Gutsmann, A.; You, S.; Richardson, A. Hsp70 and aging. Experientia. 1994, 50, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Werneburg, N.W.; Li, Z.; Bronk, S.F.; Gores, G.J. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am. J. Physiol. Gastrointest. Liver Physiol. 2006. 290, G1339–1346, 2006. [CrossRef] [PubMed]
- Yamashima, T.; Saido, T.C.; Takita, M.; Miyazawa, A.; Yamano, J.; Miyakawa, A.; Nishijyo, H.; Yamashita, J.; Kawashima, S.; Ono, T.; Yoshioka, T. Transient brain ischaemia provokes Ca2+, PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur. J. Neurosci. 1996, 8, 1932–1944. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Johnson, C.D.; Hennebelle, M.; Holtmann, T.; Taha, A.Y.; Kirpich, I.A.; Eguchi, A.; Ramsden, C.E.; Papouchado, B.G.; McClain, C.J.; Feldstein, A.E. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J. Lipid Res. 2018, 59, 1597–1609. [Google Scholar] [CrossRef]
- Shearn, C.T.; Saba, L.M.; Roede, J.R.; Orlicky, D.J.; Shearn, A.H.; Peterson, D.R. Differential carbonylation of proteins in end-stage human fatty and nonfatty NASH. Free Rad. Biol. Med. 2017, 113, 280–290. [Google Scholar] [CrossRef]
- Albano, E.; Mottaran, E.; Vidali, M.; Reale, E.; Saksena, S.; Occhino, G.; Burt, A.D.; Day, C.P. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005, 54, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Parola, M.; Alisi, A.; Marra, F.; Piemonte, F.; Mombello, C.; Sutti, S.; Povero, D.; Maina, V.; Novo, E.; Albano, E. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int. J. Mol. Med. 26, 471–476. [CrossRef] [PubMed]
- Sutti, S.; Jindal A, Locatelli, I.; Vacchiano, M.; Gigliotti, .L; Bozzola, C.; Albano, E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014, 59, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Li, Y.J.; Lovell, M.A.; Kraemer, P.J.; Gary, D.S.; Brown, R.R.; Markesbery, W.R.; Mattson, M.P. 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J. Neuropathol. Exp. Neurol. 1998, 57, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vigh L, Smith RG, Soós J, Engelhardt JI, Appel SH, Siklós L. Sublethal dose of 4-hydroxynonenal reduces intracellular calcium in surviving motor neurons in vivo. Acta Neuropathol. 2005. 109, 567-575. [CrossRef]
- Wang, Y.; Wang, W.; Yang, H.; Shao, D.; Zhao, X.; Zhang, G. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free Radic. Biol. Med. 2019, 131, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 2000, 62, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Lawless, C.; von Zglinicki, T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008, 7, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Di Naso, F.C.; Porto, R.R.; Fillmann, H.S.; Maggioni, L.; Padoin, A.V.; Ramos, R.J.; Mottin, C.C.; Bittencourt, A.; Marroni, N.A.P.; de Bittencourt, P.I.H., Jr. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring) 2015, 23, 120–129. [Google Scholar] [CrossRef]
- Eaton, S. Control of mitochondrial β-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239. [Google Scholar] [CrossRef]
- Archer, A.E.; Rogers, R.S.; Von Schulze, A.T.; Wheatley, J.L.; Morris, E.M.; McCoin, C.S.; Thyfault, J.P.; Geiger, P.C. Heat shock protein 72 regulates hepatic lipid accumulation. Heat shock protein 72 regulates hepatic lipid accumulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R696–R707. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhoven, P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- De Duve, C.; Baudhuin, P. Peroxisomes (microbodies and related particles). Physiol. Rev. 1966, 46, 323–357. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. The peroxisome: a new cytoplasmic organelle. Proc. R. Soc. Lond. B. Biol. Sci. 1969, 173, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.A.; Cochary, E.F.; Blusztajn, J.K.; Garner, S.C.; Zeisel, S.H. Accumulation of 1,2-sn-diradylglycerol with increased membrane-associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J. Biol. Chem. 1993, 268, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; da Costa, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.A.; Badea, M.; Fischer, L.M.; Zeisel, S.H. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004, 80, 163–70. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.M.; Vance, D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [CrossRef]
- Yao, Z.M.; Vance, D.E. 1989. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J. Biol. Chem. 264, 11373–11380. https://www.jbc.org/article/S0021-9258(18)60474-0/pdf.
- Zeisel, S.H.; da Costa, K.A. Choline: an essential nutrient for public health. Nutr. Rev. 2009, 67, 615–23. [Google Scholar] [CrossRef]
- Jüngst, D.; Lang, T.; Huber, P.; Lange, V.; Paumgartner, G. Effect of phospholipids and bile acids on cholesterol nucleation time and vesicular/micellar cholesterol in gallbladder bile of patients with cholesterol stones. J. Lipid Res. 1993, 34, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakamura, S.; Karoji, N.; Aikawa, T.; Suzuki, O.; Onodera, A.; Ono, Y. Hepatic function tests in heavy drinkers among workmen. Tohoku J. Exp. Med. 1967, 93, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal – A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, B.; Li, X.; Zou, Z. Role of ALDH2 in hepatic disorders: Gene polymorphism and disease pathogenesis. J. Clin. Transl.. Hepatol. 2021, 9, 90–98. [Google Scholar] [CrossRef]
- Yamashima, T. Implication of vegetable oil-derived hydroxynonenal in the lysosomal cell death for lifestyle-related diseases. nutrients 2023, 15, 609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




