Submitted:
24 January 2023
Posted:
25 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
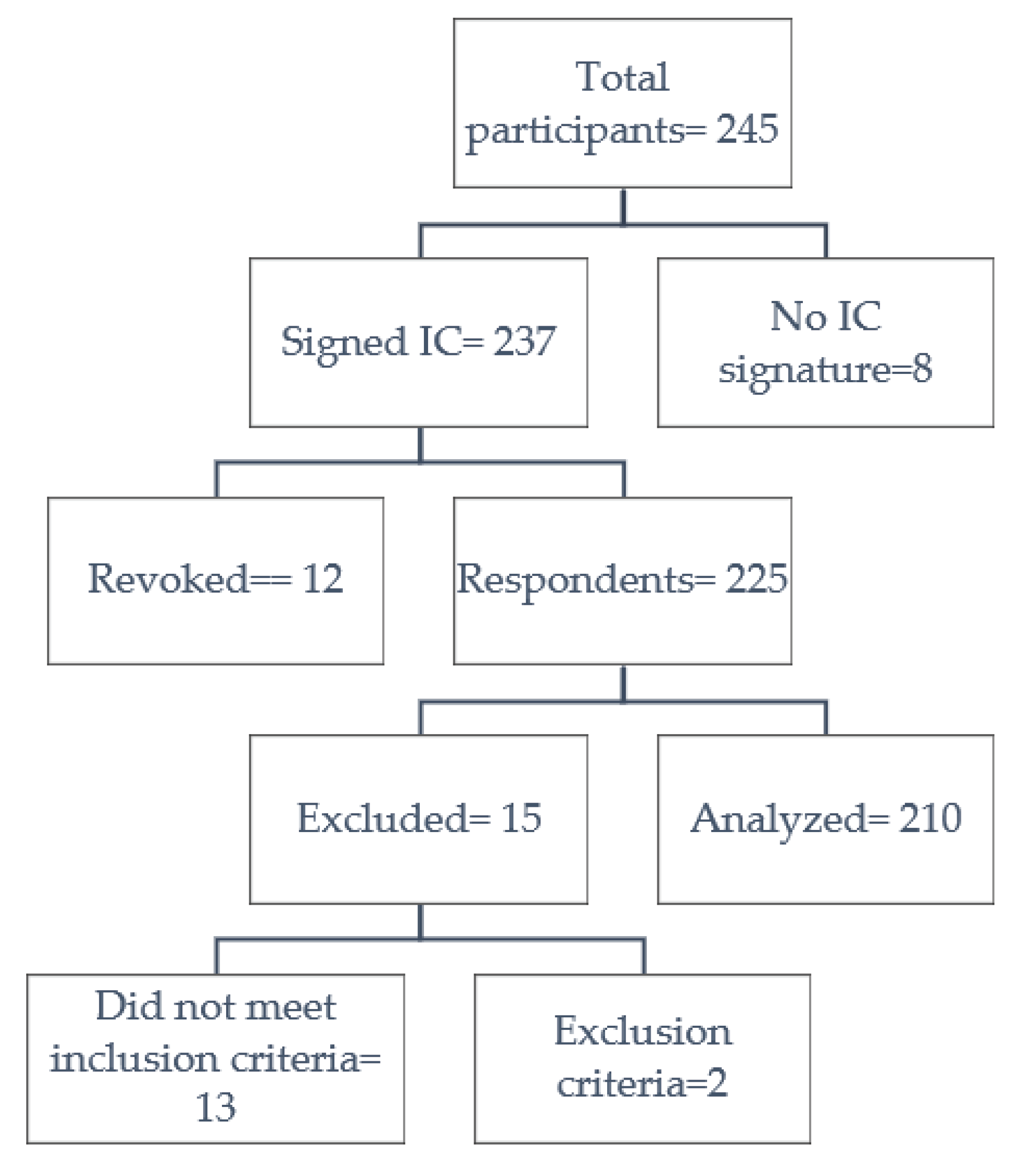
2.1. Settings, data source, and participants
2.2. Measurement of variables
2.3. Biases
2.4. Statistical analysis
3. Results
3.1. Doses administered
3.2. Vaccine administered
3.3. Group vaccine
3.4. Factors related to the occurrence of adverse events.
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| First Dose (n=210) | Second Dose (n=210) | Third Dose (n=210) | ||||||
|---|---|---|---|---|---|---|---|---|
| Oxford/AstraZeneca-Vaxzevria n (%) | Pfizer/BioNTech-Comirnaty n (%) |
Sinovac-CoronaVac n (%) |
Oxford/AstraZeneca-Vaxzevria n (%) | Pfizer/BioNTech-Comirnaty n (%) |
Sinovac-CoronaVac n (%) |
Oxford/AstraZeneca-Vaxzevria n (%) | Pfizer/BioNTech-Comirnaty n (%) |
|
| N | 32 (100.0) | 135 (100.0) | 43 (100.0) | 32 (100.0) | 135 (100.0) | 43 (100.0) | 182 (100.0) | 28 (100.0) |
| Adverse Events | 25 (78.1) | 84 (62.2) | 17 (39.5) | 17 (53.1) | 76 (56.3) | 17 (39.5) | 142 (78.0) | 16 (57.1) |
| Pain | 18 (56.3) | 74 (54.8) | 15 (34.9) | 11 (34.4) | 64 (47.4) | 16 (37.2) | 100 (54.9) | 14 (50.0) |
| Edema | 2 (6.3) | 7 (5.2) | 2 (4.7) | 0 (0.0) | 3 (2.2) | 2 (4.7) | 21 (11.5) | 3 (10.7) |
| Erythema | 2 (6.3) | 11 (8.1) | 1 (2.3) | 0 (0.0) | 3 (2.2) | 3 (7.0) | 14 (7.7) | 2 (7.1) |
| Pruritus | 1 (3.1) | 9 (6.7) | 1 (2.3) | 3 (9.4) | 6 (4.4) | 2 (4.7) | 14 (7.7) | 3 (10.7) |
| Thermal elevation | 9 (28.1) | 19 (14.1) | 2 (4.7) | 1 (3.1) | 16 (11.9) | 2 (4.7) | 81 (44.5) | 6 (21.4) |
| Diarrhea | 2 (6.3) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 12 (6.6) | 2 (7.1) |
| Nausea | 2 (6.3) | 2 (1.5) | 0 (0.0) | 1 (3.1) | 5 (3.7) | 0 (0.0) | 15 (8.2) | 0 (0.0) |
| Vomiting | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 2 (1.1) | 0 (0.0) |
| Myalgia | 14 (43.8) | 29 (21.5) | 2 (4.7) | 7 (21.9) | 26 (19.3) | 3 (7.0) | 90 (49.5) | 7 (25.0) |
| Arthralgia | 12 (37.5) | 17 (12.6) | 0 (0.0) | 5 (15.6) | 11 (8.1) | 1 (2.3) | 61 (33.5) | 4 (14.3) |
| Headache | 12 (37.5) | 24 (17.8) | 7 (16.3) | 5 (15.6) | 22 (16.3) | 3 (7.0) | 77 (42.3) | 7 (25.0) |
| Other types of adverse events |
5 (15.6) | 16 (11.9) | 0 (0.0) | 2 (6.3) | 5 (3.7) | 0 (0.0) | 20 (11.0) | 3 (10.7) |
References
- WHO. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available online: https://covid19.who.int/ (accessed on 20 April 2022).
- WHO. The World Health Report. 2006: Working Together for Health; World Health Organization, 2006. [Google Scholar]
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health 2020, 5, 3097. [Google Scholar] [CrossRef] [PubMed]
- La Federación Médica reporta decesos de profesionales de la salud por ómicron. Ecuavisa. 2022. Available online: https://www.ecuavisa.com/noticias/ecuador/la-federacion-medica-reporta-decesos-de-profesionales-de-la-salud-por-omicron-DJ1281757 (accessed on 28 July 2022).
- WHO. Access and allocation: how will there be fair and equitable allocation of limited supplies? 2021. Available online: https://www.who.int/news-room/feature-stories/detail/access-and-allocation-how-will-there-be-fair-and-equitable-allocation-of-limited-supplies (accessed on 26 July 2022).
- Ministerio de Salud Pública. Situación Epidemiológica Nacional COVID-19, Ecuador. 2022. Available online: https://www.salud.gob.ec/wp-content/uploads/2022/01/10.1.2022-epi.pdf (accessed on 28 July 2022).
- COE provincial de Pichincha. Situación cantonal por COVID-19. Distrito Metropolitano de Quito. 2021. Available online: https://coe-pichincha.senescyt.gob.ec/situacion-cantones-pichincha/ (accessed on 28 July 2022).
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. Available online: https://www.nature.com/articles/s41586-021-03398-2. [CrossRef] [PubMed]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. The Lancet 2021, 397, 2331–3. Available online: http://ees.elsevier.com/thelancet/www.thelancet.com (accessed on 21 April 2022). [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Medicine 2021, 27, 1205–11. Available online: https://www.nature.com/articles/s41591-021-01377-8 (accessed on 21 April 2022). [CrossRef] [PubMed]
- Ministerio de Salud Pública. Vacunómetro COVID-19. 2022. Available online: https://app.powerbi.com/view?r=eyJrIjoiYTkzNTFkMmUtZmUzNi00NDcwLTg0MDEtNjFkNzhhZTg5ZWYyIiwidCI6IjcwNjIyMGRiLTliMjktNGU5MS1hODI1LTI1NmIwNmQyNjlmMyJ9&pageName=ReportSection (accessed on 26 July 2022).
- CDC. Selected Adverse Events Reported after COVID-19 Vaccination|CDC. Vaccines. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 21 April 2022).
- Nohl, A.; Brune, B.; Weichert, V.; Standl, F.; Stang, A.; Dudda, M. COVID-19: Vaccination Side Effects and Sick Leave in Frontline Healthcare-Workers-A Web-Based Survey in Germany. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kitro, A.; Sirikul, W.; Thongkum, W.; Soponpong, S.; Yasamut, U.; Kiratipaisarl, W.; et al. Dynamic of anti-spike receptor binding domain (RBD) levels and short-term adverse events following a heterologous booster dose of BNT162b2 after two doses of CoronaVac in Thai health care workers. [CrossRef] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021, 9, 1255–1265. Available online: http://www.thelancet.com/article/S221326002100357X/fulltext (accessed on 21 April 2022). [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008, 61, 344–9. Available online: http://www.jclinepi.com/article/S0895435607004362/fulltext (accessed on 27 December 2022). [CrossRef] [PubMed]
- Helsinki Statement. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. the 64th WMA General Assembly, Fortaleza, Brazil, October 2013; Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 15 December 2022).
- Food and Drug Administration. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials | FDA. Center for Biologics Evaluation and Research, editor. 2007, p. 1. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (accessed on 19 April 2022).
- Organización Panamericana de la Salud. Introducción de la vacuna contra la COVID-19: Orientaciones para determinar los grupos prioritarios y elaborar la microplanificación. 2021. Available online: https://www.campusvirtualsp.org/es (accessed on 27 December 2022).
- Naito, T.; Tsuchida, N.; Kusunoki, S.; Kaneko, Y.; Tobita, M.; Hori, S.; et al. Reactogenicity and immunogenicity of BNT162b2 or mRNA-1273 COVID-19 booster vaccinations after two doses of BNT162b2 among healthcare workers in Japan: a prospective observational study. Expert Rev Vaccines 2022, 21, 1319–1229. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, I.; Bonsignore, M.; Hohenstein, S.; Bollmann, A.; Günther, R.; Kodde, C.; et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: a survey among health care workers. BMC Infect Dis. 2022, 22, 1–13. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07284-8 (accessed on 27 December 2022). [CrossRef]
- Cohen, G.; Jungsomsri, P.; Sangwongwanich, J.; Tawinprai, K.; Siripongboonsitti, T.; Porntharukchareon, T.; et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222). vaccines 2022, 18. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.v. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. International Journal of Infectious Diseases. 2021, 106, 376–381. Available online: http://www.ijidonline.com/article/S1201971221003581/fulltext (accessed on 27 December 2022). [CrossRef] [PubMed]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J Korean Med Sci. 2021, 36, 1–9 Available online:. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; et al. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open. 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Dao, T.L.; Truong, T.M.D.; Nguyen, T.H.; Phan, T.N.; Nguyen, H.M.; et al. Short-Term Adverse Effects Immediately after the Start of COVID-19 Booster Vaccination in Vietnam. Vaccines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Jokhdar, H.; Al-Tawfiq, J.A.; Al-Otaibi, S.; Assiri, A.; Almudarra, S.; et al. Adverse events following administration of COVID-19 vaccines in Saudi Arabia. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front Public Health. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Kouhpayeh, H.; Ansari, H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. Int Immunopharmacol. 2022, 109. [Google Scholar] [CrossRef] [PubMed]
| n (%) | ||
| General | 210 (100.0) | |
| Sex | Female | 138 (65.7) |
| Male | 72 (34.3) | |
| Age (Years) | Under 30 years of age | 88 (41.9) |
| 30 to 39 years old | 70 (33.3) | |
| 40 to 49 years old | 25 (11.9) | |
| 50 to 59 years old | 15 (7.1) | |
| Over 60 years old | 12 (5.7) | |
| Vaccine | Total | 630 (100.0) |
| Pfizer/BioNTech-Comirnaty | 298 (47.3) | |
| Oxford/AstraZeneca-Vaxzevria | 246 (39.0) | |
| Sinovac-CoronaVac | 86 (13.7) | |
| Total n (%) |
First Dose n (%) |
Second Dose n (%) | Third Dose n (%) |
|
|---|---|---|---|---|
| General | 630 (100.0) | 210 (33.3) | 210 (33.3) | 210 (33.4) |
| Adverse Events | 394 (62.5) | 126 (60.0) | 110 (52.4) | 158 (75.2) |
| Pain | 312 (49.5) | 107 (51.0) | 91 (43.3) | 114 (54.3) |
| Myalgia | 178 (28.3) | 45 (21.4) | 36 (17.1) | 97 (46.2) |
| Headache | 157 (24.9) | 43 (20.5) | 30 (14.3) | 84 (40.0) |
| Thermal elevation | 136 (21.6) | 30 (14.3) | 19 (9.0) | 87 (41.4) |
| Arthralgia | 111 (17.6) | 29 (13.8) | 17 (8.1) | 65 (31.0) |
| Edema | 40 (6.3) | 11 (5.2) | 5 (2.4) | 24 (11.4) |
| Pruritus | 39 (6.2) | 11 (5.2) | 11 (5.2) | 17 (8.1) |
| Erythema | 36 (5.7) | 14 (6.7) | 6 (2.9) | 16 (7.6) |
| Other types of adverse events | 100 (15.9) | 30 (3.6) | 16 (7.3) | 54 (25.8) |
| Use of medication | 305 (48.4) | 93 (44.3) | 78 (37.1) | 134 (63.8) |
| 1 drug | 243 (79.7) | 84 (90.3) | 68 (87.2) | 91 (67.9) |
| 2 drugs | 54 (17.7) | 6 (6.5) | 8 (10.3) | 40 (29.9) |
| 3 or more drugs | 8 (2.6) | 3 (3.2) | 2 (2.6) | 3 (2.2) |
| Daily Activities | 394 (100.0) | 126 (100.0) | 110 (100.0) | 158 (100.0) |
| Do not interfere with your daily activities and no treatment is administered | 158 (40.1) | 64 (50.8) | 56 (50.9) | 38 (24.1) |
| Interferes with daily activities and/or required pharmacological treatment. | 176 (44.7) | 55 (43.7) | 46 (41.8) | 75 (47.5) |
| Impedes the performance of daily activities and required treatment | 57 (14.5) | 7 (5.6) | 8 (7.3) | 42 (26.6) |
| Was hospitalized | 3(0.8) | 0 (0.0) | 0 (0.0) | 86 (1.9) |
| Oxford/AstraZeneca-Vaxzevria n (%) |
Pfizer/BioNTech-Comirnaty n (%) |
Sinovac-CoronaVac n (%) |
|
|---|---|---|---|
| General | 246 (100.0) | 298 (100.0) | 86 (100.0) |
| Adverse Events | 184 (74.8) | 176 (59.1) | 34 (39.5) |
| Pain | 129 (52.4) | 152 (51.0) | 31 (36.0) |
| Myalgia | 111 (45.1) | 62 (20.8) | 5 (5.8) |
| Headache | 94 (38.2) | 53 (17.8) | 10 (11.6) |
| Thermal elevation | 91 (37.0) | 41 (13.8) | 4 (4.7) |
| Arthralgia | 78 (31.7) | 32 (10.7) | 1 (1.2) |
| Edema | 23 (9.30) | 13 (4.4) | 4 (4.7) |
| Pruritus | 18 (7.3) | 18 (6.0) | 3 (3.5) |
| Erythema | 16 (6.5) | 16 (5.4) | 4 (4.7) |
| Other types of adverse events | 62 (22.3) | 38 (12.7) | 0 (0.0) |
| Use of medication | 155 (63.0) | 126 (42.3) | 24 (27.9) |
| 1 drug | 111 (71.6) | 110 (87.3) | 22 (91.7) |
| 2 drugs | 40 (25.8) | 12 (9.5) | 2 (8.3) |
| 3 or more drugs | 4 (2.6) | 4 (3.2) | 0 (0.0) |
| Daily Activities | 184 (100.0) | 176 (100.0) | 34 (100.0) |
| Do not interfere with your daily activities and no treatment is administered | 54 (77.0) | 79 (44.9) | 25 (73.5) |
| Interferes with daily activities and/or required pharmacological treatment. | 88 (79.0) | 79 (44.9) | 9 (26.5) |
| Impedes the performance of daily activities and required treatment | 39 (18.0) | 18 (10.2) | 0 (0.0) |
| Was hospitalized | 3 (1.6) | 0 (0.0) | 0 (0.0) |
| Heterologous n (%) |
Homologous n (%) |
|
|---|---|---|
| General | 171 (100.0) | 39 (100.0) |
| Adverse Events | 137 (80.1) | 21 (53.8) |
| Pain | 97 (56.7) | 17 (43.6) |
| Myalgia | 89 (52.0) | 8 (20.5) |
| Thermal elevation | 80 (46.8) | 7 (17.9) |
| Headache | 78 (45.6) | 6 (15.4) |
| Arthralgia | 61 (35.7) | 4 (10.3) |
| Edema | 23 (13.5) | 1 (2.6) |
| Pruritus | 15 (8.80) | 2 (5.1) |
| Erythema | 14 (8.20) | 2 (5.1) |
| Other types of adverse events | 51 (29.9) | 3 (7.7) |
| Use of medication | 120 (70.2) | 14 (35.9) |
| 1 drug | 82 (68.3) | 9 (64.3) |
| 2 drugs | 35 (29.2) | 5 (35.7) |
| 3 or more drugs | 3 (2.5) | 0 (0.0) |
| Daily Activities | 137 (100.0) | 21 (100.0) |
| Do not interfere with your daily activities and no treatment is administered | 28 (20.4) | 10 (47.6) |
| Interferes with daily activities and/or required pharmacological treatment. | 68 (49.6) | 7 (33.3) |
| Impedes the performance of daily activities and required treatment | 38 (27.7) | 4 (19.0) |
| Was hospitalized | 3 (2.2) | 0 (0.0) |
| Total (n/%) |
Adverse Event No (n/%) |
Adverse Event Yes (n/%) |
p Value | OR (CI 95%) | |
|---|---|---|---|---|---|
| General | 630(100.0) | 236 (37.5) | 394 (62.5) | - | - |
| Sex | |||||
| Female | 414 (65.7) | 136 (57.6) | 278 (70.6) | 0.001 | 1.76 (1.25 – 2.46) |
| Age | |||||
| Over 32 years old | 522 (82.9) | 193 (81.8) | 329 (83.5) | 0.579 | 1.12 (0.73 - 1.72) |
| Doses administered | |||||
| First Dose | 210 (33.3) | 84 (35.6) | 126 (32.0) | 0.353 | 0.85 (0.60 - 1.19) |
| Second Dose | 210 (33.3) | 100 (42.4) | 110 (27.9) | < 0.001 | 0.52 (0.37 - 0.74) |
| Third Dose | 210 (33.3) | 52 (22.0) | 158 (40.1) | < 0.001 | 2.36 (1.64 - 3.42) |
| Vaccine | |||||
| Pfizer/BioNTech-Comirnaty | 298 (47.3) | 122 (51.7) | 176 (44.7) | 0.087 | 1.32 (0.95 – 1.83) |
| Oxford/AstraZeneca-Vaxzevria | 246 (39.0) | 62 (26.3) | 184 (46.7) | < 0.001 | 2.45 (1.73 - 3.49) |
| Sinovac-CoronaVac | 86 (13.7) | 52 (22.0) | 34 (8.6) | < 0.001 | 0.33 (0.20 - 0.53) |
| General | 420(100.0) | 152 (36.2) | 268 (63.8) | - | - |
| Group Vaccine | |||||
| Heterologous | 171 (40.7) |
34 (22.4) |
137 (51.1) |
< 0.001 | 3.63 (2.31 – 5.69) |
| Homologous | 249 (59.3) | 118 (77.6) | 131 (48.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





