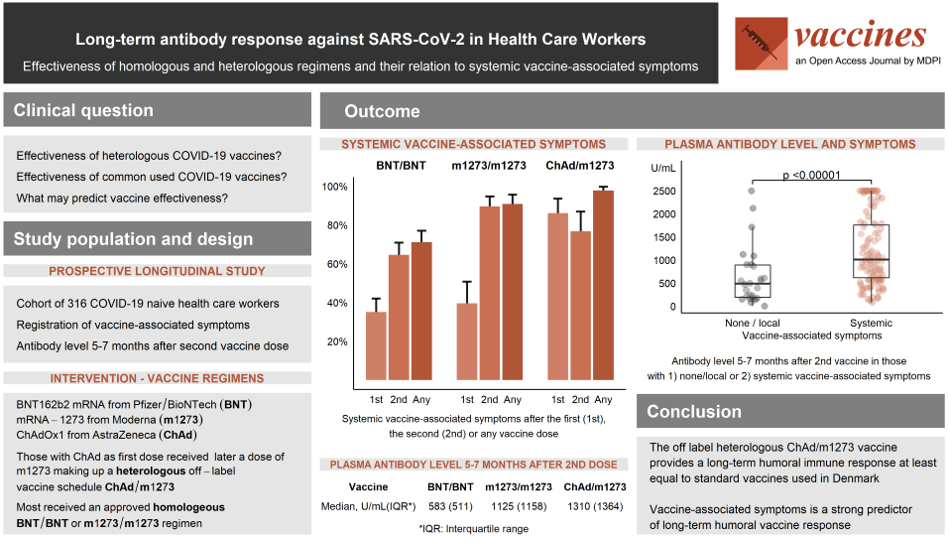
This prospective study provides data on long-term humoral immunogenicity of a heterologous off-label vaccine regimen combining the adenoviral vectored ChAdOx1 nCoV-19 from Astra-Zeneca (ChAd) with the mRNA-1273 vaccine from Moderna (m1273) in comparison to two different homologous mRNA vaccine schedules. Of the 316 COVID-19 naïve adult health care workers (HCW) included to complete a survey on vaccine-associated symptoms (VAS), 197 had received the homologous BNT162b2 mRNA vaccine from Pfizer/BioNTech (BNT/BNT), 76 the homologous m1273/m1273, and 43 the heterologous ChAd/m1273 vaccine regimen. Concentration of antibodies against SARS-CoV-2 spike protein in plasma 5-7 months after the second vaccine dose was higher in the m1273/m1273 and ChAd/m1273 than the BNT/BNT vaccine group. The frequency of systemic VAS after first vaccine dose was 86% after ChAd compared to 35% and 39% after BNT and m1273, respectively (p < 0.0001), and after second vaccine dose highest (89%) in the m1273/m1273 group (p < 0.001). Individuals with systemic VAS achieved higher levels of antibodies irrespective of vaccine regimen. In conclusion, VAS serve as a strong predictor of long-term humoral immune response, and the heterologous ChAd/m1273 vaccine regimen provides an at least equal long-term humoral immune response compared with the standard vaccine regimens used in Denmark.