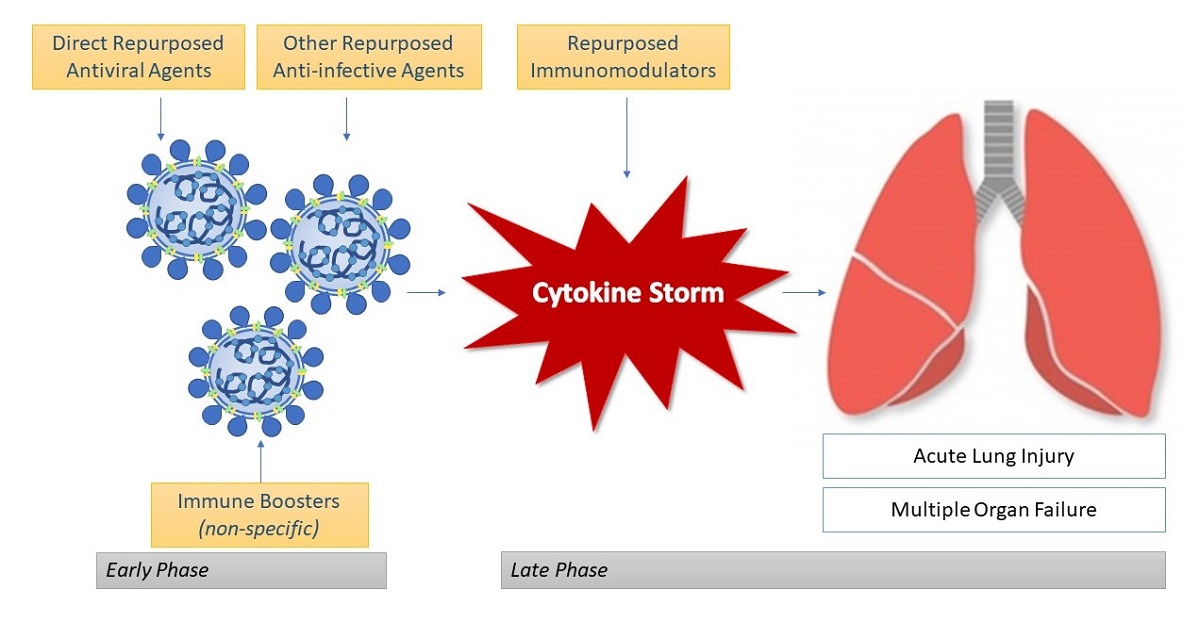
On 11 March 2020, the coronavirus disease (COVID-19) was defined by the World Health Organization as a pandemic. Severe acute respiratory syndrome-2 (SARS-CoV-2) is the newly evolving human coronavirus infection, causing (COVID-19), first appeared in Wuhan, China in December 2019 and spread rapidly all over the world. COVID-19 is being increasingly investigated in virology, epidemiology, and clinical management strategies. There is currently no established consensus on the standard of care in the pharmacological treatment of COVID-19 patients. However, certain medications suggested for other diseases tend to be potentially effective for treating this infection, although, so far, without clear evidence. Therapies include new agents which are currently tested in several clinical trials, in addition to other medications that have been repurposed as antiviral and immune-modulating therapies. Previous high-morbidity human coronavirus epidemics such as the 2003 SARS-CoV and the 2012 Middle East respiratory syndrome coronavirus (MERS-CoV) prompted the identification of compounds that could theoretically be active against the emerging coronavirus SARS-CoV-2. Moreover, the advances in molecular biology techniques and computational analysis allowed better recognition of the virus structure and quicker screening of chemical libraries to suggest potential therapies. This review aims to summarize rationalized pharmacotherapy considerations in COVID-19 patients, to serve as a tool for health care professionals at the forefront of clinical care during this pandemic. All the therapies reviewed require either additional drug development or randomized large-scale clinical trials to be justified for clinical use.