Short Note
Version 1
Preserved in Portico This version is not peer-reviewed
(E)-6-Hydroxy-2-Oxo-2H-Chromen-7-Yl 3-(4-Hydroxy-3-Methoxyphenyl)acrylate
Version 1
: Received: 8 May 2023 / Approved: 9 May 2023 / Online: 9 May 2023 (07:50:21 CEST)
A peer-reviewed article of this Preprint also exists.
Song, Y.-H. (E)-6-Hydroxy-2-oxo-2H-chromen-7-yl 3-(4-hydroxy-3-methoxyphenyl)acrylate. Molbank 2023, 2023, M1656. Song, Y.-H. (E)-6-Hydroxy-2-oxo-2H-chromen-7-yl 3-(4-hydroxy-3-methoxyphenyl)acrylate. Molbank 2023, 2023, M1656.
Abstract
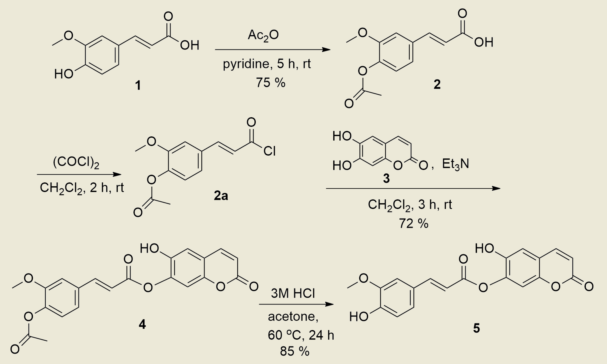
A conjugate compound 5, (E)-6-hydroxy-2-oxo-2H-chromen-7-yl 3-(4-hydroxy-3-methoxyphenyl)acrylate, of 6,7-hydroxycoumarin (esculetin) 3 and (E)-3-(4-hydroxy-3-methoxyphenyl)- acrylic acid (ferulic acid) 1 was prepared in 61% yield by the esterification reaction of a protected ferulic acid 2a with esculetin 3 in the presence of triethylamine in dichloromethane for 3 h, followed by deprotection using 3M HCl. The structure of compound 5 was confirmed by 1H, 13C NMR spectroscopy, mass-spectrometry and elemental analysis.
Keywords
coumarin; esculetin; ferulic acid; esterification; antioxidant
Subject
Chemistry and Materials Science, Organic Chemistry
Copyright: This is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Comments (0)
We encourage comments and feedback from a broad range of readers. See criteria for comments and our Diversity statement.
Leave a public commentSend a private comment to the author(s)
* All users must log in before leaving a comment