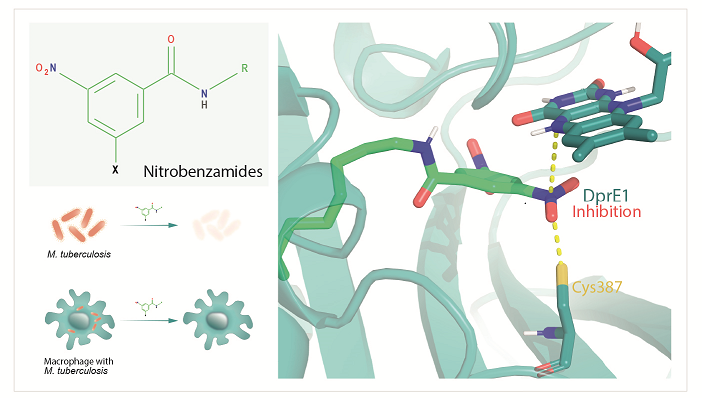
Synthesis, Activity, Toxicity, and In Silico Studies of New Antimycobacterial N-alkyl Nitrobenzamides as Potential DprE1 Inhibitors
How to cite: Pais, J.P.; Antoniuk, O.; Pires, D.; Delgado, T.; Fortuna, A.; Costa, P.J.; Anes, E.; Constantino, L. Synthesis, Activity, Toxicity, and In Silico Studies of New Antimycobacterial N-alkyl Nitrobenzamides as Potential DprE1 Inhibitors. Preprints 2024, 2024041152. https://doi.org/10.20944/preprints202404.1152.v1 Pais, J.P.; Antoniuk, O.; Pires, D.; Delgado, T.; Fortuna, A.; Costa, P.J.; Anes, E.; Constantino, L. Synthesis, Activity, Toxicity, and In Silico Studies of New Antimycobacterial N-alkyl Nitrobenzamides as Potential DprE1 Inhibitors. Preprints 2024, 2024041152. https://doi.org/10.20944/preprints202404.1152.v1
Abstract
Keywords
Subject
Copyright: This is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Comments (0)
We encourage comments and feedback from a broad range of readers. See criteria for comments and our Diversity statement.
Leave a public commentSend a private comment to the author(s)