Submitted:
28 February 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
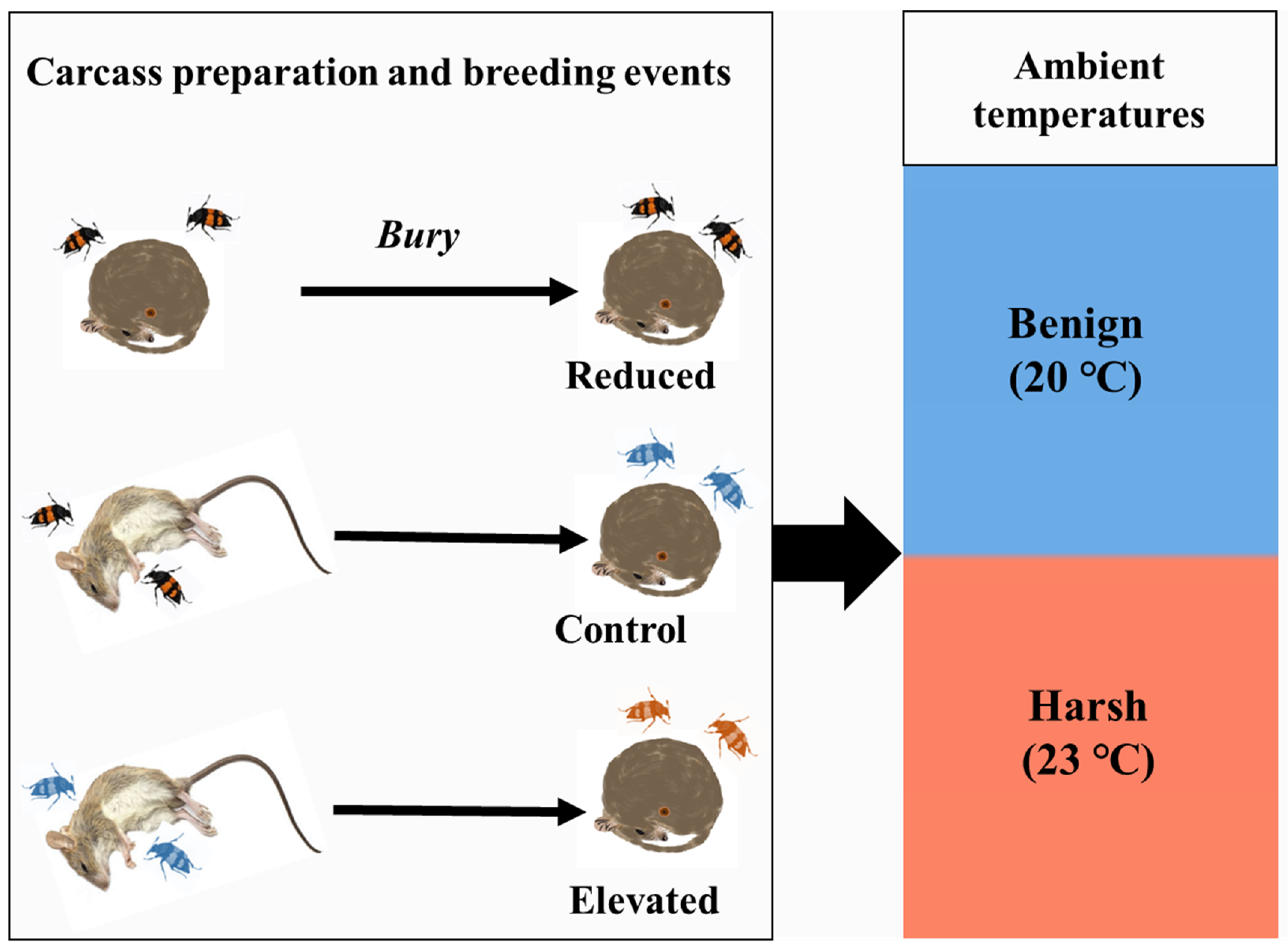
2.1. Beetles Husbandry
2.2. Experimental Protocol
2.3. Statistical Analyses
3. Results
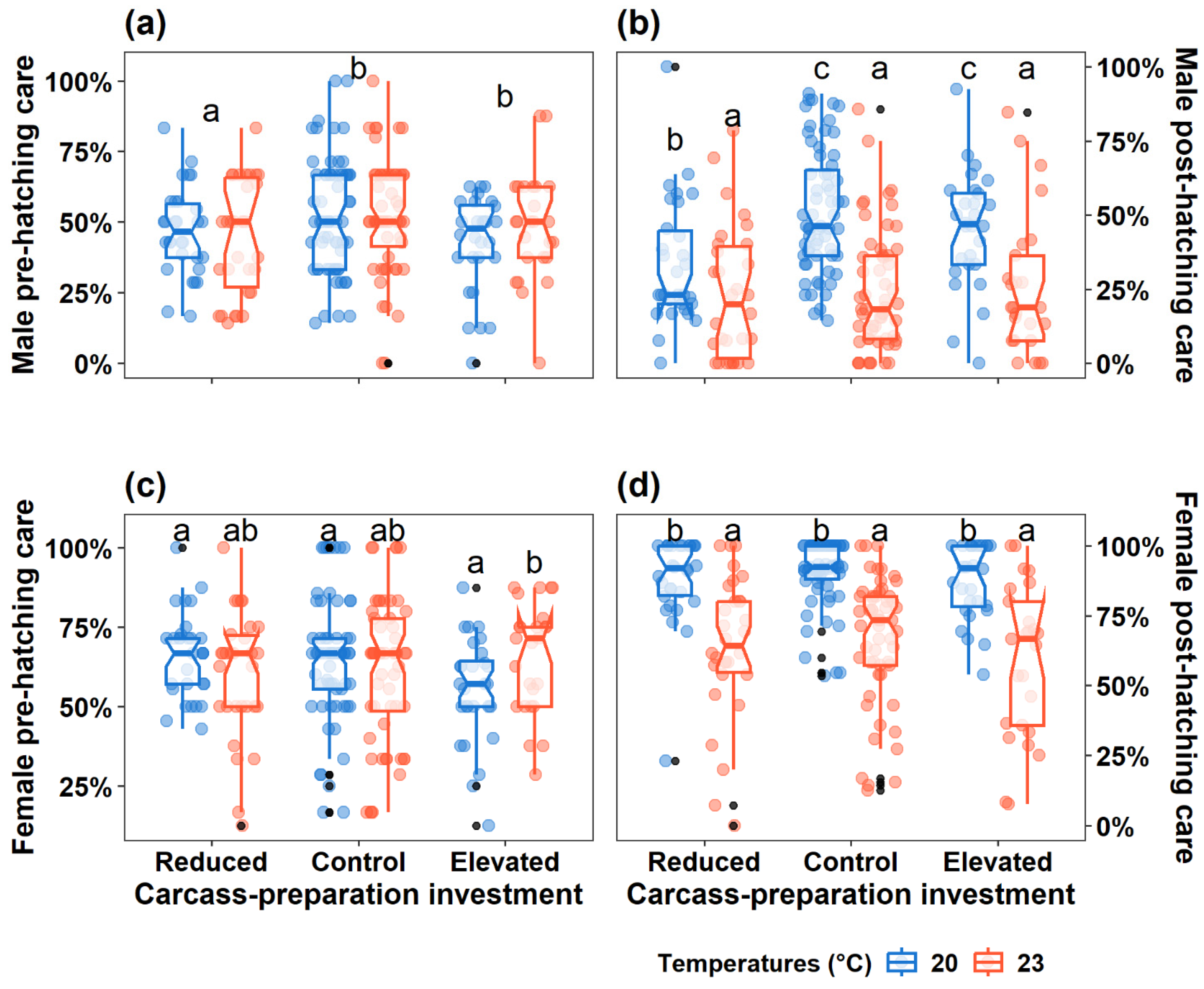
3.1. Effects of Carcass-Preparation Investment and Ambient Temperature on Parental Care
3.1.1. Male Pre- and Post-Hatching Care
3.1.2. Female Pre- and Post-Hatching Care
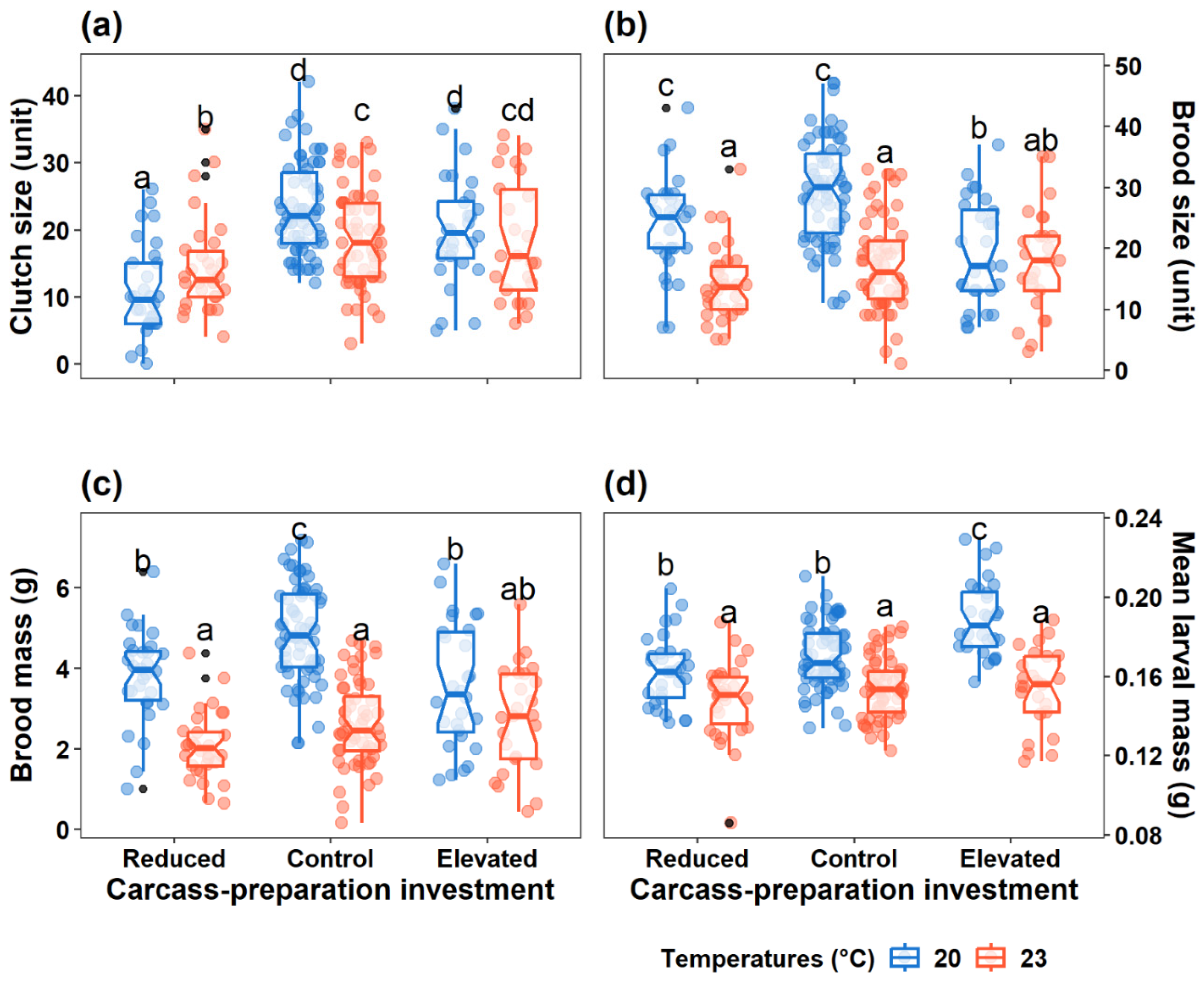
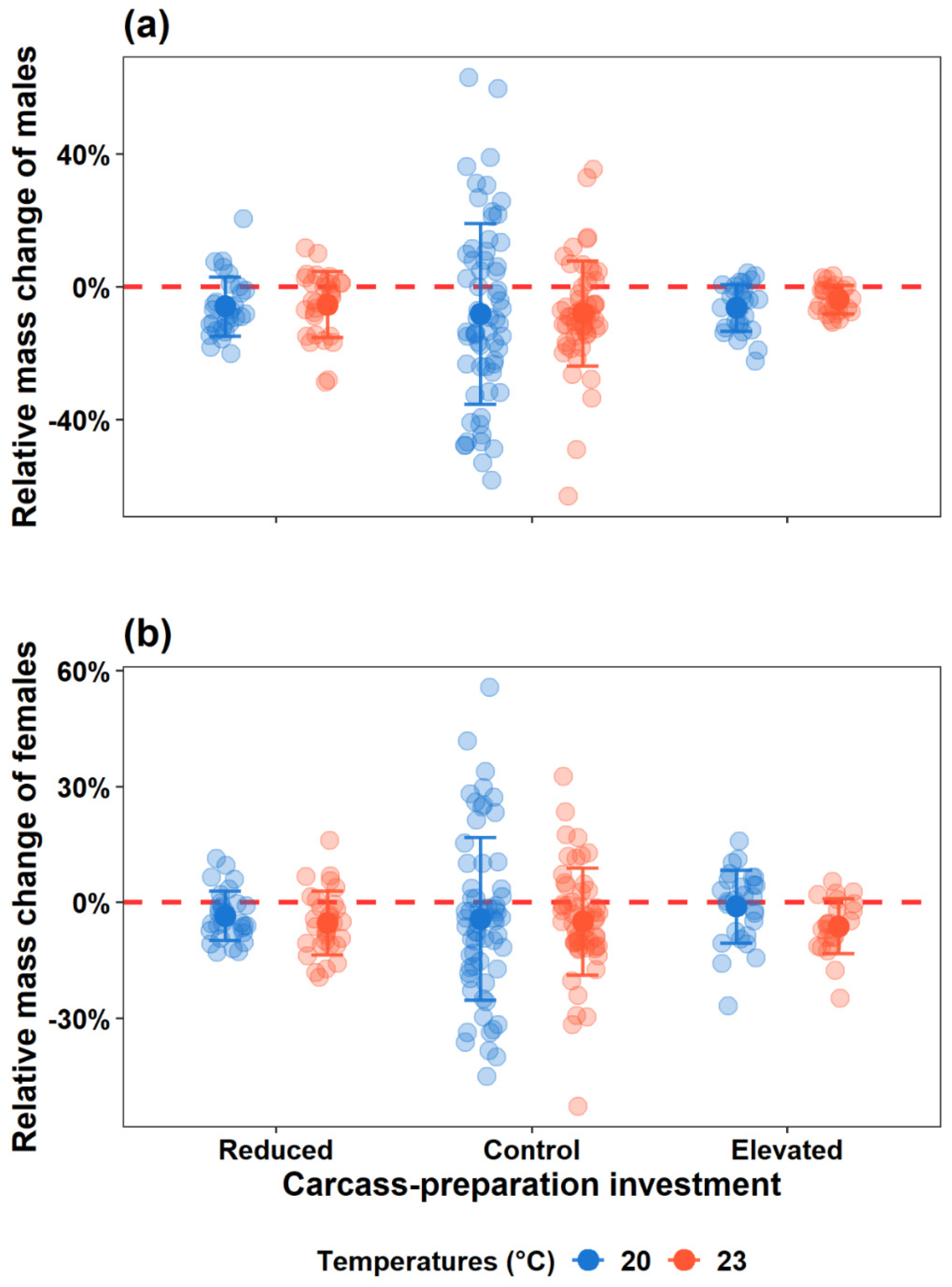
3.2. Effects of Carcass-Preparation Investment and Ambient Temperature on Reproductive Success and Parental Body Mass Change
3.2.1. Reproductive Success
3.2.2. Offspring Performance
3.2.3. Parental Body Mass Change
4. Discussion
4.1. Reduced Carcass-Preparation Investment and Ambient Temperature Impact Parental Care and Reproductive Success
4.2. Elevated Carcass-Preparation Investment and Ambient Temperature Impact Parental Care and Reproductive Success
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2023: Synthesis report. Contribution of working groups i, ii and iii to the sixth assessment report of the intergovernmental panel on climate change [Core Writing Team, H. Lee and J. Romero (Eds.)]. IPCC, Geneva, Switzerland, 2023.
- Saunders, D.A.; Mawson, P.; Dawson, R. The impact of two extreme weather events and other causes of death on Carnaby’s black cockatoo: A promise of things to come for a threatened species? Pac. Conserv. Biol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Fragueira, R.; Helfenstein, F.; Fischer, K.; Beaulieu, M. Birds of different morphs use slightly different strategies to achieve similar reproductive performance following heatwave exposure. J. Anim. Ecol. 2021, 90, 2594–2608. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Simmons, L.W. Behavioural dynamics of biparental care in the dung beetle Onthophagus taurus. Anim. Behav. 2002, 64, 65–75. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Bressac, C.; Chevrier, C. Heat stress affects male reproduction in a parasitoid wasp. J. Insect Physiol. 2013, 59, 248–254. [Google Scholar] [CrossRef]
- Pilakouta, N.; Allan, D.; Moore, E.; Russell, A.A. chronic and acute thermal stressors have non-additive effects on fertility. Proc. Royal Soc. B 2024, 291, rspb20241086. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Dickinson, M.E.; Godwin, J.L.; Lumley, A.J.; Michalczyk, Ł.; Hebberecht, L.; Thomas, P.; Franco, A.; Gage, M.J.G. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 2018, 9, 4771. [Google Scholar] [CrossRef]
- Chevrier, C.; Nguyen, T.M.; Bressac, C. Heat shock sensitivity of adult male fertility in the parasitoid wasp Anisopteromalus calandrae (Hymenoptera, Pteromalidae). J. Therm. Biol. 2019, 85, 102419. [Google Scholar] [CrossRef]
- Porcelli, D.; Gaston, K.J.; Butlin, R.K.; Snook, R.R. Local adaptation of reproductive performance during thermal stress. J. Evol. Biol. 2017, 30, 422–429. [Google Scholar] [CrossRef]
- Martinet, B.; Zambra, E.; Przybyla, K.; Lecocq, T.; Anselmo, A.; Nonclercq, D.; Rasmont, P.; Michez, D.; Hennebert, E. Mating under climate change: impact of simulated heatwaves on the reproduction of model pollinators. Funct. Ecol. 2021, 35, 739–752. [Google Scholar] [CrossRef]
- Rukke, B.A.; Sivasubramaniam, R.; Birkemoe, T.; Aak, A. Temperature stress deteriorates bed bug (Cimex lectularius) populations through decreased survival, fecundity and offspring success. PLOS ONE 2018, 13, e0193788. [Google Scholar] [CrossRef]
- McCowan, L.S.C.; Griffith, S.C. Baked Eggs: Catastrophic heatwave-induced reproductive failure in the desert-adapted Zebra finch (Taeniopygia guttata). Ibis 2021, 163, 1207–1216. [Google Scholar] [CrossRef]
- Moss, J.B.; Moore, A.J. Constrained flexibility of parental cooperation limits adaptive responses to harsh conditions. Evol. 2021, 75, 1835–1849. [Google Scholar] [CrossRef]
- Pilakouta, N.; Sellers, L.; Barratt, R.; Ligonniere, A. The consequences of heatwaves for animal reproduction are timing-dependent. Funct. Ecol. 2023, 1–9. [Google Scholar] [CrossRef]
- Wiley, E.M.; Ridley, A.R. The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 2016, 117, 187–195. [Google Scholar] [CrossRef]
- Kumar, S.; Kler, T.K.; Sekhon, G.S.; Sahni, T. Impacts on avian migratory patterns due to climate change and hormonal disruption: a review. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 69. [Google Scholar] [CrossRef]
- Both, C.; Bouwhuis, S.; Lessells, C.M.; Visser, M.E. Climate change and population declines in a long-distance migratory bird. Nature 2006, 441, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Charmantier, A.; McCleery, R.H.; Cole, L.R.; Perrins, C.; Kruuk, L.E.B.; Sheldon, B.C. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 2008, 320, 800–803. [Google Scholar] [CrossRef]
- Dawson, R.D.; Lawrie, C.C.; O’Brien, E.L. The importance of microclimate variation in determining size, growth and survival of avian offspring: experimental evidence from a cavity nesting passerine. Oecologia 2005, 144, 499–507. [Google Scholar] [CrossRef]
- McKechnie, A.E. Physiological and morphological effects of climate change. In Effects of Climate Change on Birds; 2nd ed.; Dunn, P.O., Møller, A.P., Eds, Oxford University Press: Oxford, UK, 2019; pp. 201–133. [Google Scholar]
- Sun, B.-J.; Wang, Y.; Wang, Y.; Lu, H.-L.; Du, W.-G. Anticipatory parental effects in a subtropical lizard in response to experimental warming. Front. Zool. 2018, 15, 51. [Google Scholar] [CrossRef]
- Sidhu, K.K.; Zafeiri, S.; Malcolm, C.; Caplat, P.; Lancaster, L.T.; Bocedi, G.; Pilakouta, N. Heatwaves during early development have long-term consequences for parental care in adulthood. Anim. Behav. 2024, 217, 65–72. [Google Scholar] [CrossRef]
- Walsh, B.S.; Parratt, S.R.; Hoffman, A.A.; Atkinson, D.; Snook, R.R.; Bretman, A.; Price, T.A.R. The impact of climate change on fertility. Trends Ecol. Evol. 2019, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989, 3, 259–268. [Google Scholar] [CrossRef]
- Gonzalez, O.; Zedrosser, A.; Pelletier, F.; Swenson, J.E.; Festa-Bianchet, M. Litter reductions reveal a trade-off between offspring size and number in Brown bears. Behav. Ecol. Sociobiol. 2012, 66, 1025–1032. [Google Scholar] [CrossRef]
- Richardson, J.; Stephens, J.; Smiseth, P.T. Increased allocation to reproduction reduces future competitive ability in a burying beetle. J. Anim. Ecol. 2020, 89, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.A.; Nakagawa, S. The costs of parental care: a meta-analysis of the trade-off between parental effort and survival in birds. J. Evol. Biol. 2012, 25, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Trivers, R. Parental investment and sexual selection. In sexual selection and the descent of man; Campbell, B., Eds, Aldine: Chicago, US, 1972; pp. 1871–1971. [Google Scholar]
- Coleman, R.M.; Gross, M.R. Parental investment theory: the role of past investment. Trends Ecol. Evol. 1991, 6, 404–406. [Google Scholar] [CrossRef]
- Bonnet, X.; Lourdais, O.; Shine, R.; Naulleau, G. Reproduction in a typical capital breeder: costs, currencies, and complications in the Aspic viper. Ecol. 2002, 83, 2124–2135. [Google Scholar] [CrossRef]
- van Noordwijk, A.J.; de Jong, G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986, 128, 137–142. [Google Scholar] [CrossRef]
- Glazier, D.S. Is fatter fitter? Body storage and reproduction in ten populations of the freshwater amphipod Gammarus minus. Oecologia 2000, 122, 335–345. [Google Scholar] [CrossRef]
- Messina, F.J.; Fry, J.D. Environment-dependent reversal of a life history trade-off in the seed beetle Callosobruchus maculatus. J. Evol. Biol. 2003, 16, 501–509. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press: Oxford, UK, 2009; pp.88–125.
- Scott, M.P. The ecology and behavior of burying beetles. Annu. Rev. Entomol. 1998, 43, 595–618. [Google Scholar] [CrossRef]
- Bartlett, J. The behavioural ecology of the burying beetle. Degree of Doctor of Philosophy, University of Edinburgh, Edinburgh, 1987.
- Arce, A.N.; Johnston, P.R.; Smiseth, P.T.; Rozen, D.E. Mechanisms and fitness effects of antibacterial defences in a carrion beetle. J. Evol. Biol. 2012, 25, 930–937. [Google Scholar] [CrossRef]
- Müller, J.K.; Eggert, A.-K.; Sakaluk, S.K. Carcass maintenance and biparental brood care in burying beetles: are males redundant? Ecol. Entomol. 1998, 23, 195–200. [Google Scholar] [CrossRef]
- Eggert, A.-K.; Reinking, M.; Müller, J.K. Parental care improves offspring survival and growth in burying beetles. Anim. Behav. 1998, 55, 97–107. [Google Scholar] [CrossRef]
- Schrader, M.; Hughes, P.; Jenkins, S.; Kusher, I.; Lopez, J.; Oglesby, H.; McGhee, K.E. Can age-related changes in parental care modulate inbreeding depression? A test using the burying beetle, Nicrophorus orbicollis. Ecol. Evol. 2022, 12, e9391. [Google Scholar] [CrossRef]
- Pilakouta, N.; Jamieson, S.; Moorad, J.A.; Smiseth, P.T. Parental care buffers against inbreeding depression in burying beetles. PNAS 2015, 112, 8031–8035. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.; Ashworth, C.M. Brood size and fitness in Nicrophorus Vespilloides (Coleoptera: Silphidae). Behav. Ecol. Sociobiol. 1988, 22, 429–434. [Google Scholar] [CrossRef]
- Keppner, E.M.; Laubenthal, M.; Prang, M.A.; Conrad, T.; Steiger, S. Harsh nutritional environment has positive and negative consequences for family living in a burying beetle. Ecol. Evol. 2023, 13, e9699. [Google Scholar] [CrossRef]
- Richardson, J.; Smiseth, P.T. Nutrition during sexual maturation and at the time of mating affects mating behaviour in both sexes of a burying beetle. Anim. Behav. 2019, 151, 77–85. [Google Scholar] [CrossRef]
- Richardson, J.; Smiseth, P.T. Effects of variation in resource acquisition during different stages of the life cycle on life-history traits and trade-offs in a burying beetle. J. Evol. Biol. 2019, 32, 19–30. [Google Scholar] [CrossRef]
- Eggert, A.-K.; Müller, J.K. Timing of oviposition and reproductive skew in cobreeding female burying beetles (Nicrophorus vespilloides). Behav. Ecol. 2000, 11, 357–366. [Google Scholar] [CrossRef]
- Komdeur, J.; Schrama, M.J.J.; Meijer, K.; Moore, A.J.; Beukeboom, L.W. Cobreeding in the burying beetle, Nicrophorus vespilloides: tolerance rather than cooperation. Ethol. 2013, 119, 1138–1148. [Google Scholar] [CrossRef]
- Ma, L.; Versteegh, M.A.; Hammers, M.; Komdeur, J. Sex-specific influence of communal breeding experience on parenting performance and fitness in a burying beetle. R. Soc. Open Sci. 2022, 9, 211179. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Smiseth, P.T. Maternity uncertainty in cobreeding beetles: females lay more and larger eggs and provide less care. Behav. Ecol. 2020, 31, 641–650. [Google Scholar] [CrossRef]
- Smiseth, P.T.; Dawson, C.; Varley, E.; Moore, A.J. How do caring parents respond to mate loss? differential response by males and females. Anim. Behav. 2005, 69, 551–559. [Google Scholar] [CrossRef]
- Wang, W.; Ma, L.; Versteegh, M.A.; Wu, H.; Komdeur, J. Parental care system and brood size drive sex difference in reproductive allocation: an experimental study on burying beetles. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Grew, R.; Ratz, T.; Richardson, J.; Smiseth, P. Parental care buffers against effects of ambient temperature on offspring performance in an insect. Behav. Ecol. 2019, 30, 1443–1450. [Google Scholar] [CrossRef]
- De Gasperin, O.; Kilner, R.M. Interspecific interactions change the outcome of sexual conflict over prehatching parental investment in the burying beetle Nicrophorus vespilloides. Ecol. Evol. 2015, 5, 5552–5560. [Google Scholar] [CrossRef]
- De Gasperin, O.; Duarte, A.; Troscianko, J.; Kilner, R.M. Fitness costs associated with building and maintaining the burying beetle’s carrion nest. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Temperature affects microbial decomposition of cadavers (Rattus rattus) in contrasting soils. Appl. Soil Ecol. 2008, 40, 129–137. [Google Scholar] [CrossRef]
- Mattey, S.N.; Strutt, L.; Smiseth, P.T. Intergenerational effects of inbreeding in Nicrophorus vespilloides: offspring suffer fitness costs when either they or their parents are inbred. J. Evol. Biol. 2013, 26, 843–853. [Google Scholar] [CrossRef]
- Wang, W.; Ma, L.; Versteegh, M.A.; Wu, H.; Komdeur, J. Detection of reproductive trade-offs is influenced by resource availability and maintenance: an experimental study in the burying beetle (Nicrophorus vespilloides). Behav. Ecol. Sociobiol. 2022, 76. [Google Scholar] [CrossRef]
- Bladon, E.K.; English, S.; Pascoal, S.; Kilner, R.M. Early-life effects on body size in each sex interact to determine reproductive success in the burying beetle Nicrophorus vespilloides. J. Evol. Biol. 2020, 33, 1725–1734. [Google Scholar] [CrossRef]
- Jarrett, B.J.M.; Schrader, M.; Rebar, D.; Houslay, T.M.; Kilner, R.M. Cooperative interactions within the family enhance the capacity for evolutionary change in body size. Nat. Ecol. Evol. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant graphics for data analysis. Springer: New York, US, 2016.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern applied statistics with S, 3rd ed.; Springer: New York, US, 2002. [Google Scholar]
- Hartig, F. Residual diagnostics for hierarchical (Multi-Level / Mixed) regression models [R package DHARMa version 0.4.6], 2022.
- Fox, J.; Weisberg, S.; Price, B. An R Companion to Applied Regression, 3rd ed.; Sage: Los Angeles, US, 2019.
- Lenth, R.V. emmeans: Estimated marginal means, aka least-squares means. R package version 1.7.1. R Foundation for Statistical Computing, 2021.
- Briscoe, N.J.; Morris, S.D.; Mathewson, P.D.; Buckley, L.B.; Jusup, M.; Levy, O.; Maclean, I.M.D.; Pincebourde, S.; Riddell, E.A.; Roberts, J.A.; et al. Mechanistic forecasts of species responses to climate change: the promise of biophysical ecology. Glob. Chang. Biol. 2023, 29, 1451–1470. [Google Scholar] [CrossRef]
- Lehtonen, J.; Kokko, H.; Parker, G.A. What do isogamous organisms teach us about sex and the two sexes? Philos. Trans. R. Soc. Lond. B, Biol. Sci. 2016, 371, 20150532. [Google Scholar] [CrossRef]
- Cotter, S.C.; Kilner, R.M. Sexual division of antibacterial resource defence in breeding burying beetles, Nicrophorus vespilloides. J. Anim. Ecol. 2010, 79, 35–43. [Google Scholar] [CrossRef]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Evans, R.K.; Toews, M.D.; Sial, A.A. Impact of short- and long-term heat stress on reproductive potential of drosophila Suzukii matsumura (Diptera: Drosophilidae). J. Therm. Biol. 2018, 78, 92–99. [Google Scholar] [CrossRef]
- Dowd, W.W.; King, F.A.; Denny, M.W. Thermal Variation, Thermal extremes and the physiological performance of individuals. J. Therm. Biol. 2015, 218, 1956–1967. [Google Scholar] [CrossRef]
- Malik, T.G.; Jarrett, B.J.M.; Sun, S.-J. The effect of experimental warming on reproductive performance and parental care in the burying beetle Nicrophorus nepalensis. R. Soc. Open Sci. 2024, 11, 240653. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.L.; Howard, D.R.; Hall, C.L. The thermal ecology of burying beetles: temperature influences reproduction and daily activity in Nicrophorus marginatus. Ecol. Entomol. 2021, 46, 1266–1272. [Google Scholar] [CrossRef]
- Pellissier Scott, M.; Traniello, J.F.A. Behavioural Cues Trigger ovarian development in the burying beetle, Nicrophorus tomentosus. J. Insect Physiol. 1987, 33, 693–696. [Google Scholar] [CrossRef]
| Variables | Male post-hatching care | Female pre-hatching care | Mean larval mass | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Est.± SE | z | P | Est.± SE | z | P | Est.± SE | t | P | |
| Control: 20-23 ℃ | 1.13± 0.11 | 9.84 | < 0.001 | 0.14± 0.15 | 0.97 | 0.33 | 0.02± 0.003 | 4.50 | < 0.001 |
| Reduced: 20-23 ℃ | 0.48± 0.16 | 2.95 | 0.003 | 0.23± 0.20 | 1.15 | 0.25 | 0.01± 0.005 | 3.15 | 0.002 |
| Elevated: 20-23 ℃ | 0.91± 0.17 | 5.52 | < 0.001 | -0.43± 0.20 | -2.15 | 0.03 | 0.04± 0.005 | 7.32 | < 0.001 |
| 20℃: Control-Reduced | 0.71± 0.13 | 5.33 | < 0.001 | -0.05±0.17 | -0.31 | 0.94 | 0.008± 0.004 | 1.94 | 0.11 |
| 20℃: Control-Elevated | 0.21± 0.13 | 1.69 | 0.17 | 0.36±0.17 | 2.17 | 0.06 | -0.019± 0.004 | -4.71 | < 0.001 |
| 23℃: Control-Reduced | 0.06± 0.15 | 0.41 | 0.90 | 0.03± 0.18 | 0.19 | 0.98 | 0.006± 0.004 | 1.43 | 0.28 |
| 23℃: Control-Elevated | -0.002± 0.16 | -0.01 | 1.00 | -0.21±0.19 | -1.15 | 0.44 | 0.0005± 0.004 | 0.11 | 0.99 |
| Variables | Clutch size | Brood size | Brood mass | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Est.± SE | z | P | Est.± SE | z | P | Est.± SE | t | P | |
| Control: 20-23 ℃ | 0.22± 0.07 | 2.91 | 0.004 | 0.23± 0.08 | 2.87 | 0.004 | 1.41± 0.24 | 5.89 | < 0.001 |
| Reduced: 20-23 ℃ | -0.29± 0.11 | -2.53 | 0.01 | 0.26± 0.10 | 2.52 | 0.01 | 1.00± 0.30 | 3.37 | 0.001 |
| Elevated: 20-23 ℃ | 0.08± 0.11 | 0.71 | 0.48 | -0.14± 0.11 | -1.34 | 0.19 | 0.20± 0.31 | 0.63 | 0.53 |
| 20℃: Control-Reduced | 0.76± 0.10 | 7.80 | < 0.001 | 0.08± 0.08 | 0.93 | 0.58 | 0.61± 0.25 | 2.41 | 0.03 |
| 20℃: Control-Elevated | 0.15± 0.09 | 1.59 | 0.21 | 0.32± 0.08 | 3.90 | < 0.001 | 1.03± 0.25 | 4.12 | < 0.001 |
| 23℃: Control-Reduced | 0.25± 0.10 | 2.63 | 0.02 | 0.11± 0.09 | 1.16 | 0.43 | 0.20± 0.26 | 0.79 | 0.68 |
| 23℃: Control-Elevated | 0.009± 0.10 | 0.09 | 0.99 | -0.05± 0.09 | -0.54 | 0.83 | -0.19± 0.26 | -0.72 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





