Submitted:
17 December 2024
Posted:
18 December 2024
You are already at the latest version
Abstract
Vitamin D offers a wide range of under-recognized health benefits beyond its well-established role in musculoskeletal health. It plays a crucial role in extra-renal and skeletal tissues, prenatal and newborn health, brain health, immune function, cancer prevention, cardiovascular disease, etc. Current clinical guidelines, particularly the Endocrine Society's 2024 recommendations, remain limited in scope and have not addressed the vital extra-skeletal benefits of this vitamin nor the thresholds for vitamin D assays. Their recommendations were based on conclusions from randomized controlled trials of the benefits of vitamin D, which were infrequently found. Most such trials included participants with above average 25-hydroxyvitamin D [25(OH)D] concentrations and treated with low vitamin D doses and analyzed based on intention to treat. This review considers the role of vitamin D in reducing the risk of incidence and death for eight of the top ten causes of death in the US illustrating that serum concentrations above 30 ng/mL (75 nmol/L) compared to <20 ng/mL are associated with significantly reduced risk of incidence and mortality rates for many health outcomes. Since about a quarter of the US population and 60% in Central Europe have 25(OH)D concentrations <20 ng/mL, significant reductions in disease rates and deaths could be achieved by raising those values above the minimum of 30 ng/mL. Daily vitamin D supplementation with 2000 international units (IU) (50 µg) of vitamin D3 is recommended for prevention of vitamin D deficiency/insufficiency (i.e, serum 25(OH)D < 30 ng/mL)—sufficient for musculoskeletal system functions. However, intake above 4000 IU/day are recommended to raise serum 25(OH)D to the range 40‒70 ng/mL to achieve protection against many adverse health outcomes. This review aims to pave the way for more inclusive, evidence-based guidelines that enhance public health and personalized care.
Keywords:
1. Introduction
1.1. Global Vitamin D Deficiency
1.2. Alternative Strategies to Randomized Controlled Trials Better Suited for Nutrients
1.3. Hypovitaminosis Increases Vulnerability to Diseases—Causality
2. Health Benefits of Vitamin D
2.1. Cardiovascular Disease
2.2. Stroke
2.3. Cancer Prevention
2.4. Immune System Support and COVID-19
2.5. Chronic Lower Respiratory Diseases
2.6. Alzheimer’s Disease and Dementia
2.7. Type 2 Diabetes Mellitus
2.8. Chronic Kidney Disease
2.9. Chronic liver disease
2.10. Bone and Oral Health
2.11. Autoimmune Diseases
2.12. Pregnancy, Birth, and Infancy Outcomes
2.13. All-Cause Mortality
2.14. Vitamin D-Deficiency Associated Deaths and Their Prevention
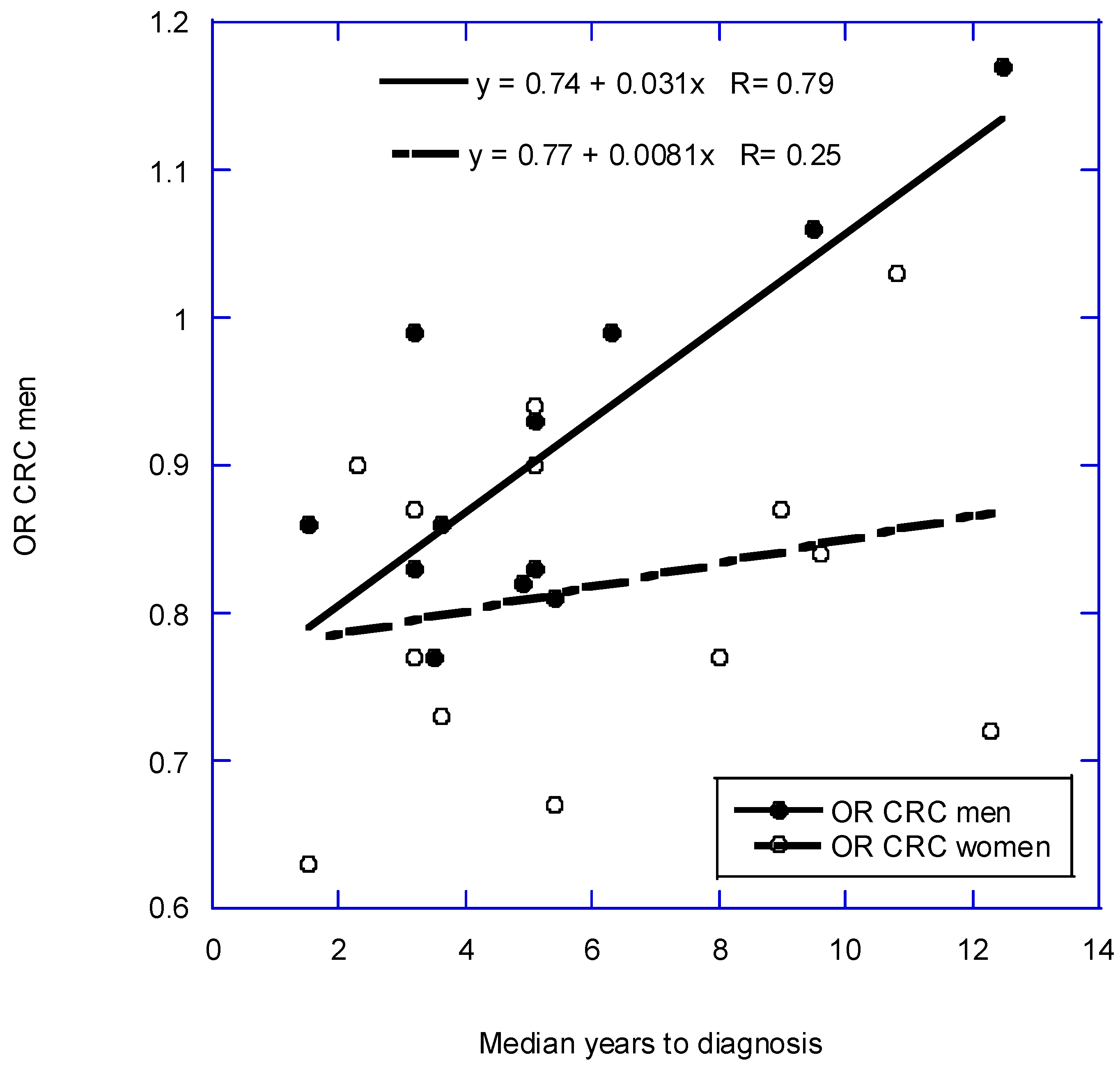
2.15. Racial Disparities
2.16. Higher vitamin D doses and serum 25(OH)D concentrations from recommendations
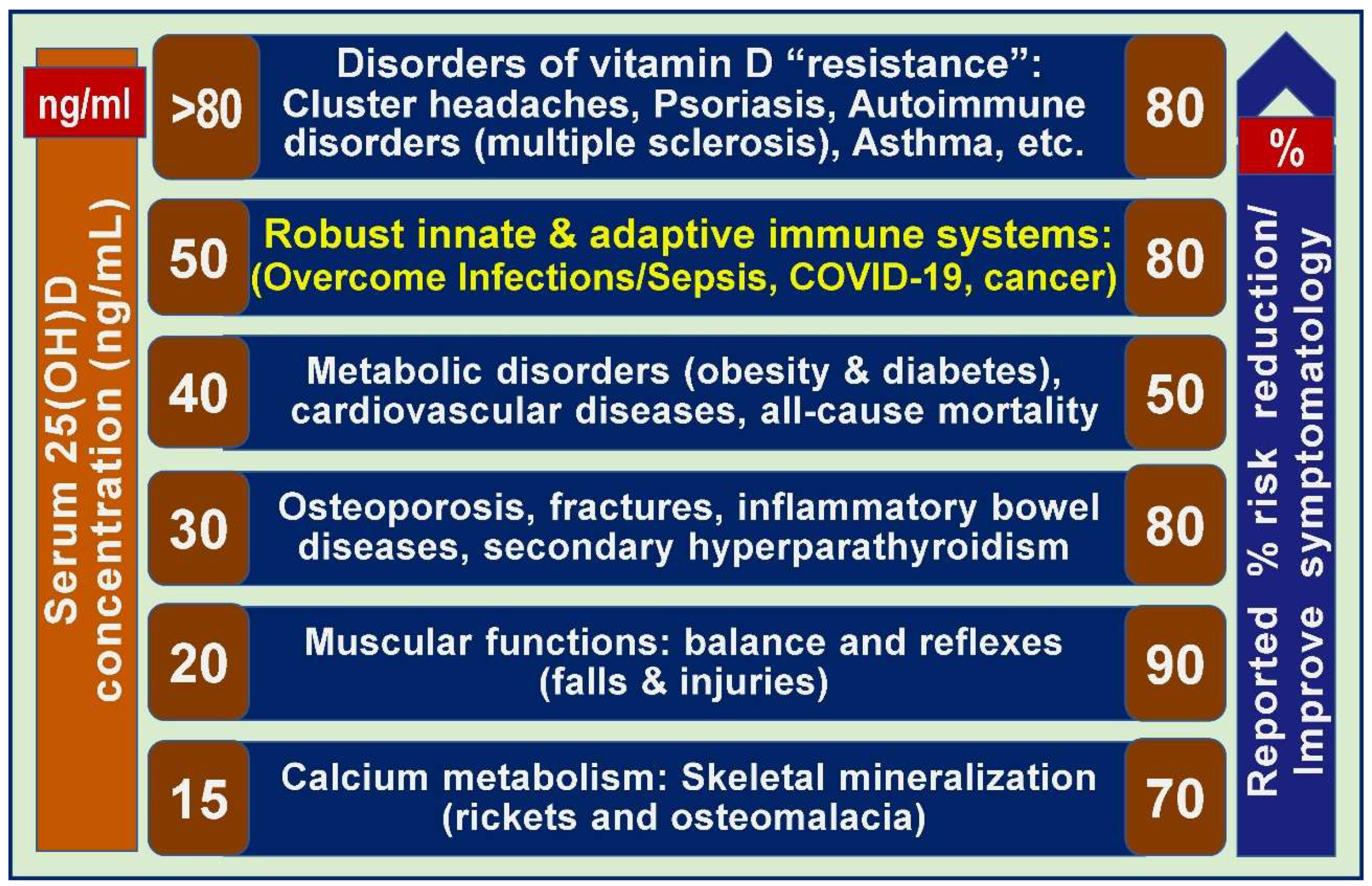
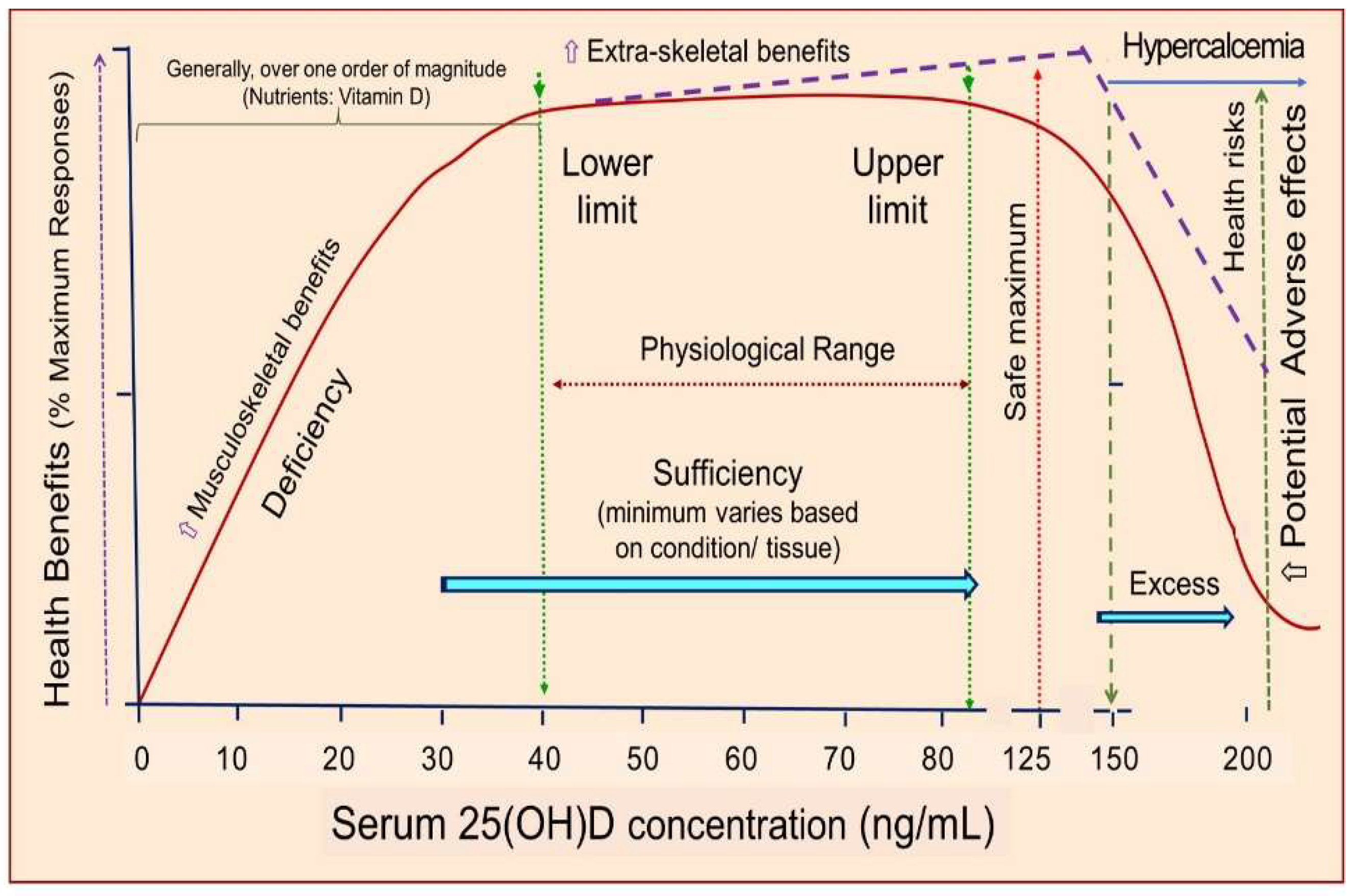
2.17. Different serum 25(OH)D concentrations are needed to overcome diverse disorders
2.18. High-Dose Vitamin D and Vitamin D Resistance
3. Recommendations for Prevention of Vitamin D Deficiency
4. Critiques of the Endocrine Society’s Vitamin D Guideline
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S., et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2024, 109, 1907-1947. [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911-1930. [CrossRef]
- Wimalawansa, S.J.; Weiss, S.T.; Hollis, B.W. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients 2024, 16, 3969. [CrossRef]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16. [CrossRef]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [CrossRef]
- Wimalawansa, S.J. Controlling Chronic Diseases and Acute Infections with Vitamin D Sufficiency. Nutrients 2023, 15. [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011, 96, 53-58. [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D'Agostino, D., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019, 380, 33-44. [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R., et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med 2019, 381, 520-530. [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol 2017, 5, 986-1004. [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14. [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14. [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014, 72, 48-54. [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J Clin Endocrinol Metab 2018, 103, 2936-2948. [CrossRef]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, 2977. [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 1980, 9, 227-231. [CrossRef]
- Clarke, R.; Shipley, M.; Lewington, S.; Youngman, L.; Collins, R.; Marmot, M.; Peto, R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999, 150, 341-353. [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D., et al. Circulating vitamin D and colorectal cancer risk: An international pooling Project of 17 cohorts. J Natl Cancer Inst 2019, 111, 158-169. [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [CrossRef]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc R Soc Med 1965, 58, 295-300.
- Doll, R. Proof of causality: deduction from epidemiological observation. Perspect Biol Med 2002, 45, 499-515. [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2021, 115, 154434. [CrossRef]
- Wimalawansa, S.J. Unlocking insights: Navigating COVID-19 challenges and Emulating future pandemic Resilience strategies with strengthening natural immunity. Heliyon 2024, 10, e34691. [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D's Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci Rep 2019, 9, 17685. [CrossRef]
- Kochanek, K.D.; Murphy, S.L.; Xu, J.Q.; Arias, E. Mortality in the United States, 2022. National Center for Health Statistics: Hayettsville, MD, 2024; 10.15620/cec.135850.
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L., et al. 3. Prevention or Delay of Type 2 Diabetes and Associated Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S41-S48. [CrossRef]
- Heart Disease Facts. Availabe online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 1 December 2024).
- Wang, Y.; Wang, X.; Wang, C.; Zhou, J. Global, Regional, and National Burden of Cardiovascular Disease, 1990-2021: Results From the 2021 Global Burden of Disease Study. Cureus 2024, 16, e74333. [CrossRef]
- Gardner, D.G.; Chen, S.; Glenn, D.J. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol 2013, 305, R969-977. [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J Steroid Biochem Mol Biol 2018, 175, 29-43. [CrossRef]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2018, 5, 87. [CrossRef]
- Camici, M.; Galetta, F.; Franzoni, F.; Carpi, A.; Zangeneh, F. Vitamin D and heart. Intern Emerg Med 2013, 8 Suppl 1, S5-9. [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol Res 2018, 129, 95-99. [CrossRef]
- Ye, H.; Li, Y.; Liu, S.; Zhang, X.; Liang, H.; Wang, Y.; Wang, R.; Liu, H.; Wen, Y.; Jing, C., et al. Association between serum 25-hydroxyvitamin D and vitamin D dietary supplementation and risk of all-cause and cardiovascular mortality among adults with hypertension. Nutr J 2024, 23, 33. [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L., et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol 2019, 4, 765-776. [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G., et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ 2023, 381, e075230. [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293-2309. [CrossRef]
- Bahadorpour, S.; Hajhashemy, Z.; Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: a systematic review and dose-response meta-analysis of epidemiologic studies. Nutr Rev 2022, 81, 1-25. [CrossRef]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J Endocr Soc 2021, 5, bvab124. [CrossRef]
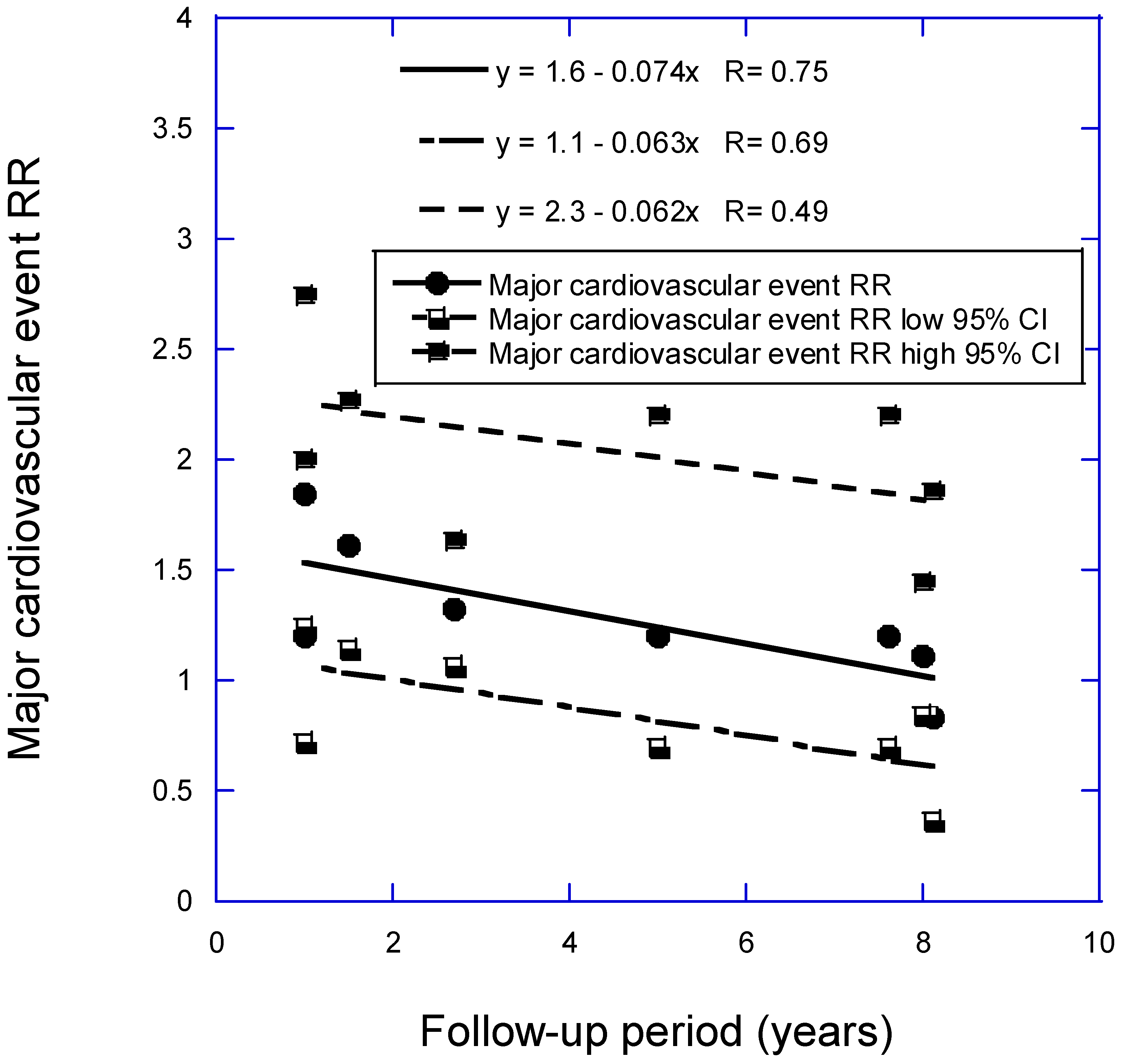
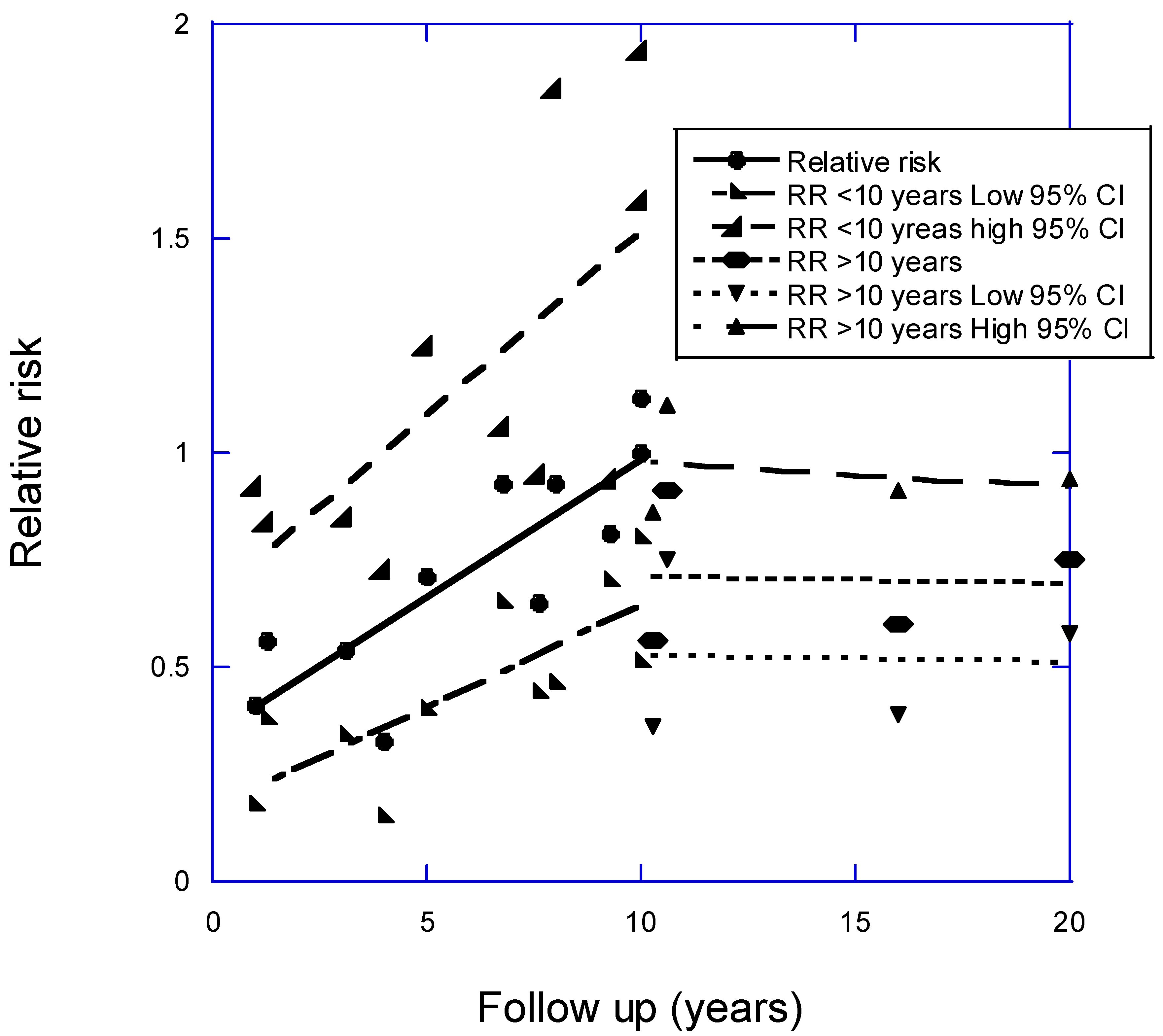
- Grant, W.B.; Boucher, B.J. How Follow-Up Period in Prospective Cohort Studies Affects Relationship Between Baseline Serum 25(OH)D Concentration and Risk of Stroke and Major Cardiovascular Events. Nutrients 2024, 16. [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hypponen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J 2022, 43, 1731-1739. [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; al., e. 2023 Heart Disease and Stroke Statistics Update Fact Sheet. Availabe online: https://professional.heart.org/-/media/PHD-Files-2/Science-News/2/2023-Heart-and-Stroke-Stat-Update/2023-Statistics-At-A-Glance-final_1_17_23.pdf (accessed on December 1, 2024).
- Su, C.; Jin, B.; Xia, H.; Zhao, K. Association between Vitamin D and Risk of Stroke: A PRISMA-Compliant Systematic Review and Meta-Analysis. Eur Neurol 2021, 84, 399-408. [CrossRef]
- Xiong, J.; Zhao, C.; Li, J.; Li, Y. A systematic review and meta-analysis of the linkage between low vitamin D and the risk as well as the prognosis of stroke. Brain Behav 2024, 14, e3577. [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12-49. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209-249. [CrossRef]
- Jorde, R.; Sneve, M.; Hutchinson, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 2010, 171, 903-908. [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS One 2018, 13, e0199265. [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007, 85, 1586-1591. [CrossRef]
- Lappe, J.; Garland, C.; Gorham, E. Vitamin D Supplementation and Cancer Risk. JAMA 2017, 318, 299-300. [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E., et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized clinical trial. JAMA Netw Open 2020, 3, e2025850. [CrossRef]
- Garcia, A.M.; Bishop, E.L.; Li, D.; Jeffery, L.E.; Garten, A.; Thakker, A.; Certo, M.; Mauro, C.; Tennant, D.A.; Dimeloe, S., et al. Tolerogenic effects of 1,25-dihydroxyvitamin D on dendritic cells involve induction of fatty acid synthesis. J Steroid Biochem Mol Biol 2021, 211, 105891. [CrossRef]
- Abo-Zaid, M.A.; Hamdi, H.A.; Elashmawy, N.F. Vitamin D and Immunity: A comprehensive review of its impact on autoimmunity, allergy suppression, antimicrobial defense, and cancer inhibition. Egypt J Immunol 2023, 30, 47-66.
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr Osteoporos Rep 2022, 20, 186-193. [CrossRef]
- Engin, M.M.N.; Ozdemir, O. Role of vitamin D in COVID-19 and other viral infections. World J Virol 2024, 13, 95349. [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One 2020, 15, e0239252. [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Dominguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest 2022, 45, 167-179. [CrossRef]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine (Baltimore) 2019, 98, e17252. [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [CrossRef]
- Grant, W.B.; Giovannucci, E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States. Dermatoendocrinol 2009, 1, 215-219. [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010, 91, 1255-1260. [CrossRef]
- Pal, R.; Banerjee, M.; Bhadada, S.K.; Shetty, A.J.; Singh, B.; Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest 2022, 45, 53-68. [CrossRef]
- Mostert, S.; Hoogland, M.; Huibers, M.; Kasperss, G. Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022. BMJ Public Health 2024, 2, e000282. [CrossRef]
- Fortner, A.; Schumacher, D. First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization. Discoveries (Craiova) 2021, 9, e122. [CrossRef]
- Emergency Use Authorization for Vaccines Explained. Availabe online: https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained (accessed on December 1, 2024).
- Hilser, J.R.; Spencer, N.J.; Afshari, K.; Gilliland, F.D.; Hu, H.; Deb, A.; Lusis, A.J.; Tang, W.H.W.; Hartiala, J.A.; Hazen, S.L., et al. COVID-19 Is a Coronary Artery Disease Risk Equivalent and Exhibits a Genetic Interaction With ABO Blood Type. Arterioscler Thromb Vasc Biol 2024, 44, 2321-2333. [CrossRef]
- Mantilla-Beniers, N.B.; Bjornstad, O.N.; Grenfell, B.T.; Rohani, P. Decreasing stochasticity through enhanced seasonality in measles epidemics. J R Soc Interface 2010, 7, 727-739. [CrossRef]
- Pomeroy, L.W.; Magsi, S.; McGill, S.; Wheeler, C.E. Mumps epidemic dynamics in the United States before vaccination (1923-1932). Epidemics 2023, 44, 100700. [CrossRef]
- Rozhnova, G.; Metcalf, C.J.; Grenfell, B.T. Characterizing the dynamics of rubella relative to measles: the role of stochasticity. J R Soc Interface 2013, 10, 20130643. [CrossRef]
- Bloom-Feshbach, K.; Alonso, W.J.; Charu, V.; Tamerius, J.; Simonsen, L.; Miller, M.A.; Viboud, C. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One 2013, 8, e54445. [CrossRef]
- Martinez, M.E. The calendar of epidemics: Seasonal cycles of infectious diseases. PLoS Pathog 2018, 14, e1007327. [CrossRef]
- Shaman, J.; Goldstein, E.; Lipsitch, M. Absolute humidity and pandemic versus epidemic influenza. Am J Epidemiol 2011, 173, 127-135. [CrossRef]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007, 85, 860-868. [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E., et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One 2015, 10, e0118108. [CrossRef]
- Roe, K. The epithelial cell types and their multi-phased defenses against fungi and other pathogens. Clin Chim Acta 2024, 563, 119889. [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770-1773. [CrossRef]
- COPD. Availabe online: https://www.cdc.gov/cdi/indicator-definitions/chronic-obstructive-pulmonary-disease.html (accessed on 26 November 2024).
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; Unit, N.R.G.R.H. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022, 10, 447-458. [CrossRef]
- Zhu, Z.; Wan, X.; Liu, J.; Zhang, D.; Luo, P.; Du, W.; Chen, L.; Su, J.; Hang, D.; Zhou, J., et al. Vitamin D status and chronic obstructive pulmonary disease risk: a prospective UK Biobank study. BMJ Open Respir Res 2023, 10. [CrossRef]
- Lu, K.; Tan, J.S.; Li, T.Q.; Yuan, J.; Wang, H.; Wang, W. An inverse causal association between genetically predicted vitamin D and chronic obstructive pulmonary disease risk. Front Nutr 2023, 10, 1111950. [CrossRef]
- Fu, L.; Fei, J.; Tan, Z.X.; Chen, Y.H.; Hu, B.; Xiang, H.X.; Zhao, H.; Xu, D.X. Low Vitamin D Status Is Associated with Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J Immunol 2021, 206, 515-523. [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 2021, 10.1038/s41574-021-00593-z. [CrossRef]
- Gustavsson, A.; Norton, N.; Fast, T.; Frolich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T., et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement 2023, 19, 658-670. [CrossRef]
- Castelli, V.; Cimini, A.; Ferri, C. Cytokine Storm in COVID-19: "When You Come Out of the Storm, You Won't Be the Same Person Who Walked in". Front Immunol 2020, 11, 2132. [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G., et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimers Dement 2023, 19, 2389-2396. [CrossRef]
- Yao, L.; Chen, M.; Zhang, N.; Ma, S.; Xie, X.; Xu, S.; Nie, Z.; Wang, W.; Zhou, E.; Xu, S., et al. The Mediation Role of Sleep Disturbances between Vitamin D and Depressive Symptoms: A Cross-Sectional Study. Brain Sci 2023, 13. [CrossRef]
- Zhao, W.; Zhu, D.M.; Shen, Y.; Zhang, Y.; Chen, T.; Cai, H.; Zhu, J.; Yu, Y. The protective effect of vitamin D supplementation as adjunctive therapy to antidepressants on brain structural and functional connectivity of patients with major depressive disorder: a randomized controlled trial. Psychol Med 2024, 54, 2403-2413. [CrossRef]
- Roy, N.M.; Al-Harthi, L.; Sampat, N.; Al-Mujaini, R.; Mahadevan, S.; Al Adawi, S.; Essa, M.M.; Al Subhi, L.; Al-Balushi, B.; Qoronfleh, M.W. Impact of vitamin D on neurocognitive function in dementia, depression, schizophrenia and ADHD. Front Biosci (Landmark Ed) 2021, 26, 566-611. [CrossRef]
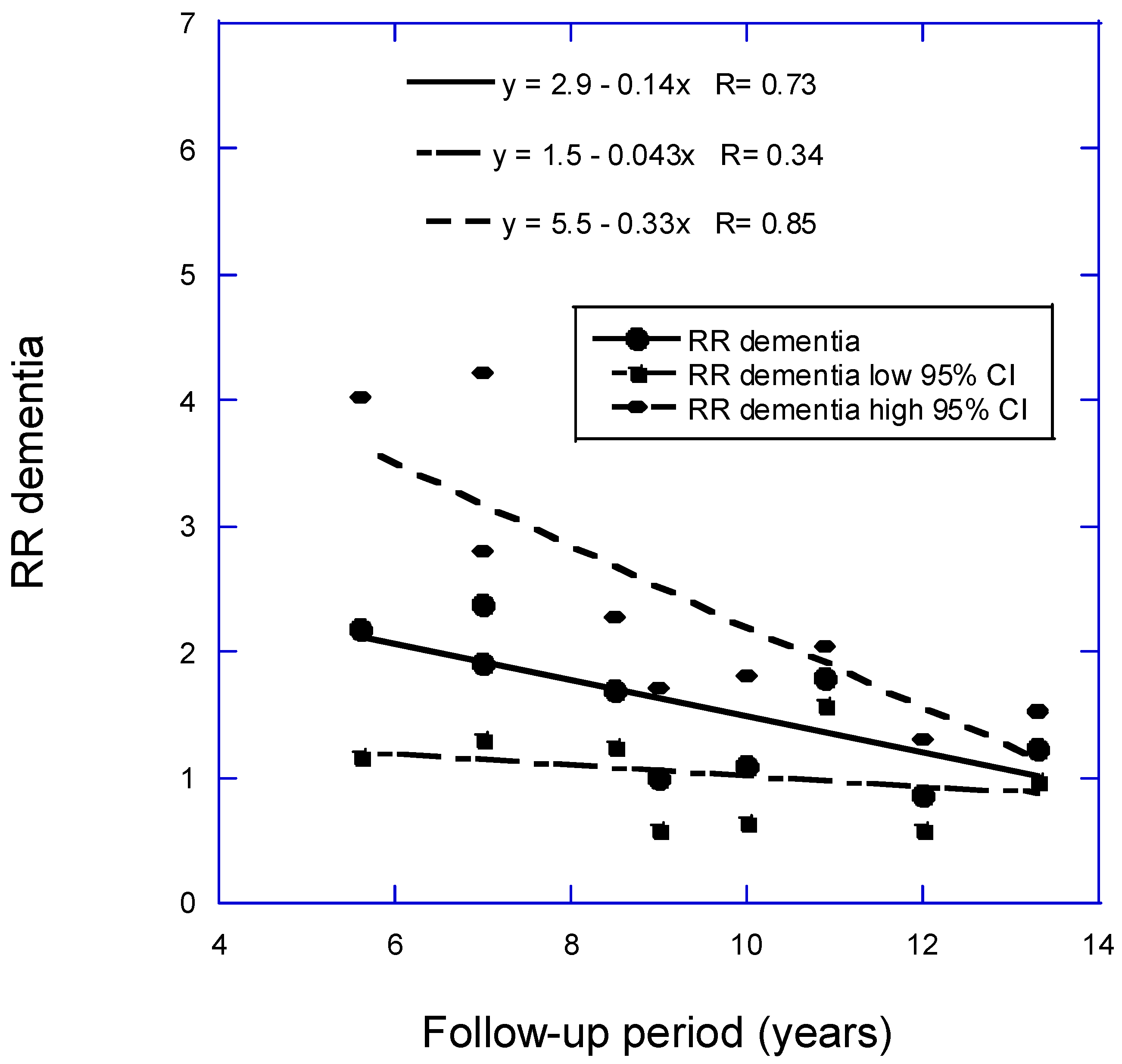
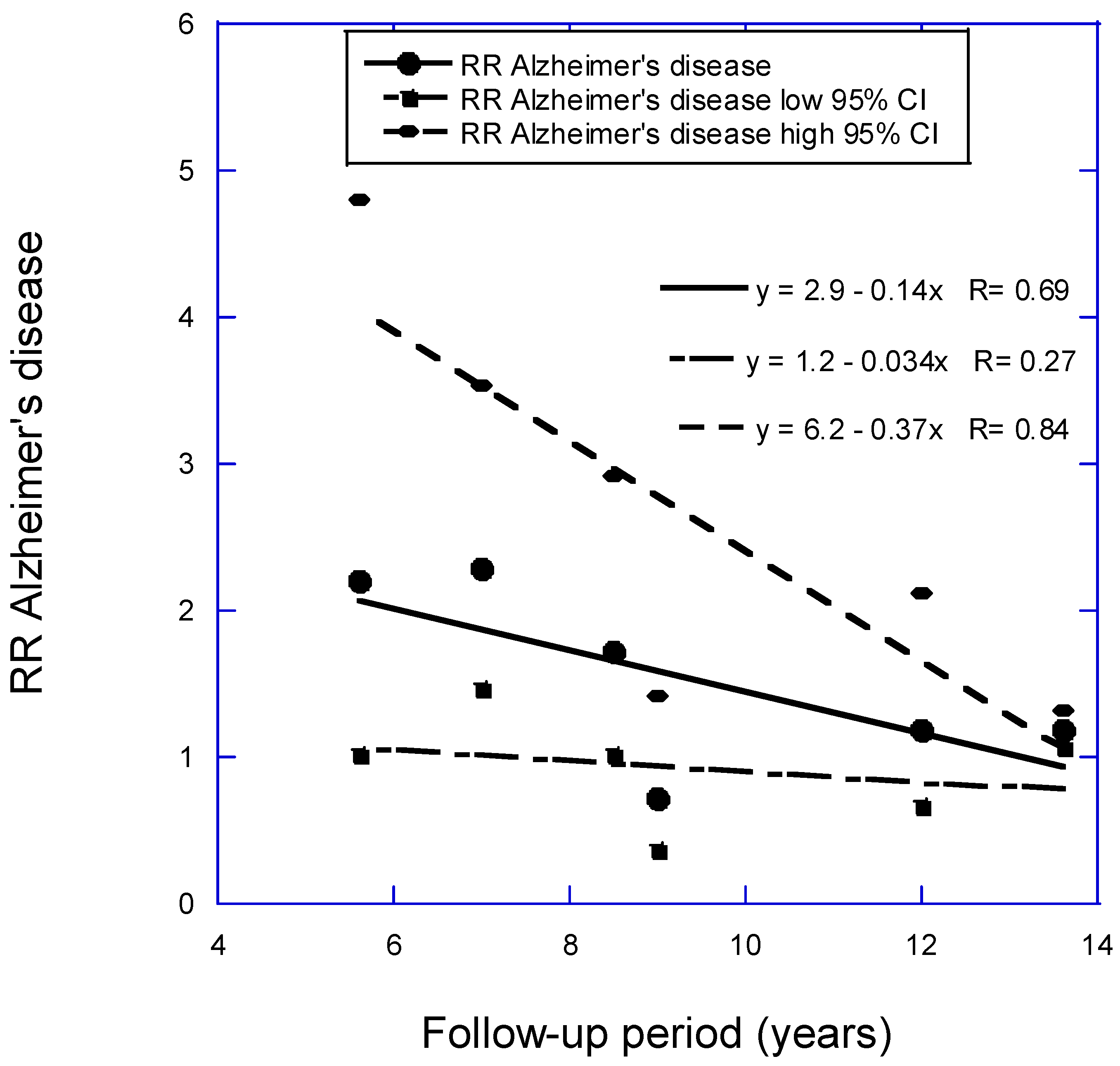
- Grant, W.B. Follow-Up Period Affects the Association between Serum 25-Hydroxyvitamin D Concentration and Incidence of Dementia, Alzheimer's Disease, and Cognitive Impairment. Nutrients 2024, 16, 3211. [CrossRef]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci 2020, 10. [CrossRef]
- Gezen-Ak, D.; Dursun, E. Vitamin D, a Secosteroid Hormone and Its Multifunctional Receptor, Vitamin D Receptor, in Alzheimer’s Type Neurodegeneration. J. Alz. Disease 2023, 95, 1273-1299. [CrossRef]
- Abboud, M. Vitamin D Supplementation and Sleep: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2022, 14. [CrossRef]
- Mergl, R.; Dogan-Sander, E.; Willenberg, A.; Wirkner, K.; Kratzsch, J.; Riedel-Heller, S.; Allgaier, A.K.; Hegerl, U.; Sander, C. The effect of depressive symptomatology on the association of vitamin D and sleep. BMC Psychiatry 2021, 21, 178. [CrossRef]
- Shuai, J.; Gao, M.; Zou, Q.; He, Y. Association between vitamin D, depression, and sleep health in the National Health and Nutrition Examination Surveys: a mediation analysis. Nutr Neurosci 2024, 27, 934-941. [CrossRef]
- Mokry, L.E.; Ross, S.; Morris, J.A.; Manousaki, D.; Forgetta, V.; Richards, J.B. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology 2016, 87, 2567-2574. [CrossRef]
- Navale, S.S.; Mulugeta, A.; Zhou, A.; Llewellyn, D.J.; Hypponen, E. Vitamin D and brain health: an observational and Mendelian randomization study. Am J Clin Nutr 2022, 116, 531-540. [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. JAMA 2021, 326, 1-13. [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017, 128, 40-50. [CrossRef]
- He, L.P.; Li, C.P.; Liu, C.W.; Gu, W. The Regulatory Effect of Vitamin D on Pancreatic Beta Cell Secretion in Patients with Type 2 Diabetes. Curr Med Chem 2024, 10.2174/0109298673270429240805050928. [CrossRef]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10. [CrossRef]
- Oliveira, I.N.N.; Macedo-Silva, A.; Coutinho-Cruz, L.; Sanchez-Almeida, J.; Tavares, M.P.S.; Majerowicz, D. Effects of vitamin D supplementation on metabolic syndrome parameters in patients with obesity or diabetes in Brazil, Europe, and the United States: A systematic review and meta-analysis. J Steroid Biochem Mol Biol 2024, 243, 106582. [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int J Mol Sci 2023, 24. [CrossRef]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; Group, D.d.R. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916-2922. [CrossRef]
- Wan, Z.; Guo, J.; Pan, A.; Chen, C.; Liu, L.; Liu, G. Association of Serum 25-Hydroxyvitamin D Concentrations With All-Cause and Cause-Specific Mortality Among Individuals With Diabetes. Diabetes Care 2021, 44, 350-357. [CrossRef]
- Rohold, C.K.; Jorgensen, H.L.; Vojdeman, F.J.; Madsen, C.M.; Olsen, A.; Heegaard, A.M.; Lind, B.S.; Tjonneland, A.; Schwarz, P.; Gaede, P.H. Levels of plasma 25-hydroxy vitamin D and risk of developing type 2 diabetes in a large Danish primary health care population. Acta Diabetol 2024, 10.1007/s00592-024-02368-0. [CrossRef]
- Murphy, D.; McCulloch, C.E.; Lin, F.; Banerjee, T.; Bragg-Gresham, J.L.; Eberhardt, M.S.; Morgenstern, H.; Pavkov, M.E.; Saran, R.; Powe, N.R., et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med 2016, 165, 473-481. [CrossRef]
- McCullough, K.P.; Morgenstern, H.; Saran, R.; Herman, W.H.; Robinson, B.M. Projecting ESRD Incidence and Prevalence in the United States through 2030. J Am Soc Nephrol 2019, 30, 127-135. [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019, 96, 1048-1050. [CrossRef]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662-664. [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022, 12, 7-11. [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int 2008, 73, 1355-1363. [CrossRef]
- Li, R.; Li, Y.; Fan, Z.; Liu, Z.; Lin, J.; He, M. L-shaped association of serum 25-hydroxyvitamin D with all-cause and cardiovascular mortality in older people with chronic kidney disease: results from the NHANES database prospective cohort study. BMC Public Health 2023, 23, 1260. [CrossRef]
- Chronic Liver Disease/Cirrhosis Mortality by State. Availabe online: https://www.cdc.gov/nchs/pressroom/sosmap/liver_disease_mortality/liver_disease.htm (accessed on December 1, 2024).
- Yang, F.; Ren, H.; Gao, Y.; Zhu, Y.; Huang, W. The value of severe vitamin D deficiency in predicting the mortality risk of patients with liver cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol 2019, 43, 722-729. [CrossRef]
- Ravaioli, F.; Pivetti, A.; Di Marco, L.; Chrysanthi, C.; Frassanito, G.; Pambianco, M.; Sicuro, C.; Gualandi, N.; Guasconi, T.; Pecchini, M., et al. Role of Vitamin D in Liver Disease and Complications of Advanced Chronic Liver Disease. Int J Mol Sci 2022, 23. [CrossRef]
- Holick, M.F. The One-Hundred-Year Anniversary of the Discovery of the Sunshine Vitamin D(3): Historical, Personal Experience and Evidence-Based Perspectives. Nutrients 2023, 15. [CrossRef]
- Kazemian, E.; Pourali, A.; Sedaghat, F.; Karimi, M.; Basirat, V.; Sajadi Hezaveh, Z.; Davoodi, S.H.; Holick, M.F. Effect of supplemental vitamin D3 on bone mineral density: a systematic review and meta-analysis. Nutr Rev 2023, 81, 511-530. [CrossRef]
- Manoj, P.; Derwin, R.; George, S. What is the impact of daily oral supplementation of vitamin D3 (cholecalciferol) plus calcium on the incidence of hip fracture in older people? A systematic review and meta-analysis. Int J Older People Nurs 2023, 18, e12492. [CrossRef]
- Hujoel, P.P. Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev 2013, 71, 88-97. [CrossRef]
- Lu, E.M. The role of vitamin D in periodontal health and disease. J Periodontal Res 2023, 58, 213-224. [CrossRef]
- Amon, U.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Safety Data in Patients with Autoimmune Diseases during Treatment with High Doses of Vitamin D3 According to the "Coimbra Protocol". Nutrients 2022, 14. [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11. [CrossRef]
- Marinho, A.; Carvalho, C.; Boleixa, D.; Bettencourt, A.; Leal, B.; Guimaraes, J.; Neves, E.; Oliveira, J.C.; Almeida, I.; Farinha, F., et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3(+)/IL-17A ratio and clinical course in systemic lupus erythematosus patients: a study in a Portuguese cohort. Immunol Res 2017, 65, 197-206. [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T., et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet 2023, 402, 1261-1271. [CrossRef]
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O'Brien, M.J., et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011-2019. JAMA 2021, 326, 660-669. [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol 2020, 76, 1690-1702. [CrossRef]
- Xiao, M.Z.X.; Whitney, D.; Guo, N.; Bentley, J.; Shaw, G.M.; Druzin, M.L.; Butwick, A.J. Trends in eclampsia in the United States, 2009-2017: a population-based study. J Hypertens 2022, 40, 490-497. [CrossRef]
- Arshad, R.; Sameen, A.; Murtaza, M.A.; Sharif, H.R.; Iahtisham Ul, H.; Dawood, S.; Ahmed, Z.; Nemat, A.; Manzoor, M.F. Impact of vitamin D on maternal and fetal health: A review. Food Sci Nutr 2022, 10, 3230-3240. [CrossRef]
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14. [CrossRef]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F., et al. Maternal 25(OH)D concentrations >/=40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS One 2017, 12, e0180483. [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front Endocrinol (Lausanne) 2018, 9, 500. [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W., et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017, 12, e0170791. [CrossRef]
- Phillips, D.; Barker, G.E.; Brewer, K.M. Christmas and New Year as risk factors for death. Soc Sci Med 2010, 71, 1463-1471. [CrossRef]
- Grant, W.B.; Bhattoa, H.P.; Boucher, B.J. Seasonal variations of U.S. mortality rates: Roles of solar ultraviolet-B doses, vitamin D, gene exp ression, and infections. J Steroid Biochem Mol Biol 2017, 173, 5-12. [CrossRef]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D(3) supplementation can have important patient and public health benefits. Eur J Clin Nutr 2020, 74, 366-376. [CrossRef]
- Gospodarska, E.; Ghosh Dastidar, R.; Carlberg, C. Intervention Approaches in Studying the Response to Vitamin D(3) Supplementation. Nutrients 2023, 15. [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010-2022. Am J Prev Med 2024, 66, 582-589. [CrossRef]
- Ahmad, F.B.; Cisewski, J.A.; Xu, J.; Anderson, R.N. COVID-19 Mortality Update - United States, 2022. MMWR Morb Mortal Wkly Rep 2023, 72, 493-496. [CrossRef]
- Gwira, J.A.; Fryar, C.D.; gu, Q. Prevalence of Total, Diagnosed, and Undiagnosed Diabetes in Adults: United States, August 2021–August 2023. Atlanta, GA, 2024.
- Hu, C.; Yang, M. Trends of serum 25(OH) vitamin D and association with cardiovascular disease and all-cause mortality: from NHANES survey cycles 2001-2018. Front Nutr 2024, 11, 1328136. [CrossRef]
- Ames, B.N.; Grant, W.B.; Willett, W.C. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 2021, 13. [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K., et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev 2013, 12, 976-989. [CrossRef]
- Pludowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokol, D.; Czech-Kowalska, J.; Debski, R.; Decsi, T.; Dobrzanska, A.; Franek, E., et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol 2013, 64, 319-327. [CrossRef]
- Holick, M.F. Vitamin D requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int 1998, 8 Suppl 2, S24-29. [CrossRef]
- Heaney, R.P. Toward a physiological referent for the vitamin D requirement. J Endocrinol Invest 2014, 37, 1127-1130. [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J Steroid Biochem Mol Biol 2019, 189, 228-239. [CrossRef]
- Hill, A.; Starchl, C.; Dresen, E.; Stoppe, C.; Amrein, K. An update of the effects of vitamins D and C in critical illness. Front Med (Lausanne) 2022, 9, 1083760. [CrossRef]
- Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 microg) of Vitamin D for Adults in the General Population. Nutrients 2024, 16, 391. [CrossRef]
- Pludowski, P.; Marcinowska-Suchowierska, E.; Togizbayev, G.; Belaya, Z.; Grant, W.B.; Pilz, S.; Holick, M.F. Daily and Weekly "High Doses" of Cholecalciferol for the Prevention and Treatment of Vitamin D Deficiency for Obese or Multi-Morbidity and Multi-Treatment Patients Requiring Multi-Drugs-A Narrative Review. Nutrients 2024, 16, 2541. [CrossRef]
- Holick, M.F. Revisiting Vitamin D Guidelines: A Critical Appraisal of the Literature. Endocr Pract 2024, 10.1016/j.eprac.2024.10.011. [CrossRef]
- Cheng, R.Z. Key Differences Between Conventional Nutrition and Orthomolecular Nutrition: The Role of Dosing and Regulatory Challenges. Availabe online: https://orthomolecular.org/resources/omns/v20n21.shtml (accessed on 7 December 2024).
- Ghanaati, S.; Choukroun, J.; Volz, U.; Hueber, R.; Mourão, C.F.A.B.; Sader, R.; Kawase-Koga, Y.; Mazhari, R.; Amrein, K.; Maybohm, P., et al. One Hundred Years after Vitamin D Discovery: Is There Clinical Evidence for Supplementation Doses? International Journal of Growth Factors and Stem Cells in Dentistry 2020, 3, 3-11. [CrossRef]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A. 25-Hydroxyvitamin D response to graded vitamin D(3) supplementation among obese adults. J Clin Endocrinol Metab 2013, 98, 4845-4851. [CrossRef]
- He, C.S.; Fraser, W.D.; Tang, J.; Brown, K.; Renwick, S.; Rudland-Thomas, J.; Teah, J.; Tanqueray, E.; Gleeson, M. The effect of 14 weeks of vitamin D3 supplementation on antimicrobial peptides and proteins in athletes. J Sports Sci 2016, 34, 67-74. [CrossRef]
- Kimball, S.M.; Mirhosseini, N.; Holick, M.F. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermatoendocrinolgy 2018, 9, e1300213. [CrossRef]
- Pittas, A.; Dawson-Hughes, B.; Staten, M. Vitamin D Supplementation and Prevention of Type 2 Diabetes. Reply. N Engl J Med 2019, 381, 1785-1786. [CrossRef]
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. [CrossRef]
- Johnson, K.C.; Pittas, A.G.; Margolis, K.L.; Peters, A.L.; Phillips, L.S.; Vickery, E.M.; Nelson, J.; Sheehan, P.R.; Reboussin, D.; Malozowski, S., et al. Safety and tolerability of high-dose daily vitamin D(3) supplementation in the vitamin D and type 2 diabetes (D2d) study-a randomized trial in persons with prediabetes. Eur J Clin Nutr 2022, 76, 1117-1124. [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of vitamin D's calcemic activity and non-calcemic genomic activity and Individual tesponsiveness: A randomized controlled double-blind clinical trial. Sci Rep 2019, 9, 17685. [CrossRef]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol 2018, 175, 60-81. [CrossRef]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 2010, 152, 307-314. [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010, 10, 482-496. [CrossRef]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: a controlled study. J Sci Med Sport 2014, 17, 8-12. [CrossRef]
- Khan, S.R.; Whiteman, D.C.; Kimlin, M.G.; Janda, M.; Clarke, M.W.; Lucas, R.M.; Neale, R.E. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: a pilot randomised controlled trial. Photochem Photobiol Sci 2018, 10.1039/c7pp00378a. [CrossRef]
- Armas, L.A.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004, 89, 5387-5391. [CrossRef]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr Osteoporos Rep 2012, 10, 4-15. [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234-1243. [CrossRef]
- Whitford, G., Pashley, DH, Stringer, GI. Fluoride renal clearance: A pH-dependent event. Am J Physiol Whitford GM, Pashley DH, Stringer GI1976, 230, 527-532.
- Perez-Lopez, F.R. Vitamin D and its implications for musculoskeletal health in women: an update. Maturitas 2007, 58, 117-137. [CrossRef]
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol 2014, 3, 29-33. [CrossRef]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control 2012, 23, 363-370. [CrossRef]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K., et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health 2014, 104, e43-50. [CrossRef]
- Cauley, J.A.; LaCroix, A.Z.; Wu, L.; Horwitz, M.; Danielson, M.E.; Bauer, D.C.; Lee, J.S.; Jackson, R.D.; Robbins, J.A.; Wu, C., et al. Serum 25 hydroxyvitamin D concentrations and the risk of hip Fractures: The women's health initiative. Annals of internal medicine 2008, 149, 242-250.
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr 2011, 65, 1016-1026. [CrossRef]
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-hydroxyvitamin D values and risk of all-cause and cause-specific mortality: A population-based cohort study. Mayo Clin Proc 2018, 93, 721-730. [CrossRef]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [CrossRef]
- Cheng, R.Z. Understanding and Addressing Vitamin D Resistance: A Comprehensive Approach Integrating Genetic, Environmental, and Nutritional Factors. Availabe online: https://orthomolecular.org/resources/omns/v20n13.shtml (accessed on 1 December 2024).
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front Immunol 2021, 12, 655739. [CrossRef]
- Jarvelin, U.M.; Jarvelin, J.M. Significance of vitamin D responsiveness on the etiology of vitamin D-related diseases. Steroids 2024, 207, 109437. [CrossRef]
- AlGhamdi, S.; AlHarthi, H.; Khoja, S.; AlJefri, A.; AlShaibi, H.F. A High Dose, Not Low Dose, of Vitamin D Ameliorates Insulin Resistance in Saudi Women. J Clin Med 2022, 11. [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr 2011, 14, 340-346. [CrossRef]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482-495. [CrossRef]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003, 77, 204-210. [CrossRef]
- Pilz, S.; Marz, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C., et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front Endocrinol (Lausanne) 2018, 9, 373. [CrossRef]
- Cashman, K.D.; O'Neill, C.M. Strategic food vehicles for vitamin D fortification and effects on vitamin D status: A systematic review and meta-analysis of randomised controlled trials. J Steroid Biochem Mol Biol 2024, 238, 106448. [CrossRef]
- Jaaskelainen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.; Kroger, H.; Karppinen, J., et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am J Clin Nutr 2017, 105, 1512-1520. [CrossRef]
- Ikonen, H.; Lumme, J.; Seppala, J.; Pesonen, P.; Piltonen, T.; Jarvelin, M.R.; Herzig, K.H.; Miettunen, J.; Niinimaki, M.; Palaniswamy, S., et al. The determinants and longitudinal changes in vitamin D status in middle-age: a Northern Finland Birth Cohort 1966 study. Eur J Nutr 2021, 60, 4541-4553. [CrossRef]
- Pludowski, P.; Kos-Kudla, B.; Walczak, M.; Fal, A.; Zozulinska-Ziolkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewinski, A., et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [CrossRef]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001, 358, 1500-1503. [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985, 76, 1536-1538. [CrossRef]
- Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Policola, C.; Della Casa, S.; Pontecorvi, A.; Giaccari, A. 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 2010, 18, 1906-1910. [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.D.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G., et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Algheimer's Dement. 2022, 10.1002/alz.12836, 1-8. [CrossRef]
- Wakeman, M. A Literature Review of the Potential Impact of Medication on Vitamin D Status. Risk Manag Healthc Policy 2021, 14, 3357-3381. [CrossRef]
- Grant, W.B.; Fakhoury, H.M.A.; Karras, S.N.; Al Anouti, F.; Bhattoa, H.P. Variations in 25-Hydroxyvitamin D in Countries from the Middle East and Europe: The Roles of UVB Exposure and Diet. Nutrients 2019, 11, 2065. [CrossRef]
| Age range | All-cause Mortality rate (deaths/ 100,000) in 2022 [25] |
CVD* Mortality rate (deaths/ 100,000) in 2022 [138] |
Cancer Incidence (%) 2017‒2019 [45] |
COVID-19 Mortality rate (deaths/ 100,000) in 2022 [139] |
DM Prevalence (%) Aug. 2021‒ Aug 2023 [140] |
|---|---|---|---|---|---|
| 25-34 | 163 | 0‒49 years 3.5 |
5 | 20‒39 years, 3.6 |
|
| 35-44 | 255 | 65 | 12 | ||
| 45-54 | 453 | 50‒64 years F, 10.8; M, 11.8 |
30 | 40‒59 years 17.7 |
|
| 55-64 | 992 | 251 | 71 | ||
| 65-74 | 1979 | 541 | F, 24.3; M 31.9 | 158 | 60+ years 27.3 |
| 75-84 | 4706 | 495 | 414 | ||
| 85+ | 14,390 | 698 | F, 39.6; M, 41.6 | 1224 |
| Year | Organization, country | Vitamin D Dose (IU/day) |
Serum 25(OH)D (ng/mL) |
Health basis | Comments | Reference |
|---|---|---|---|---|---|---|
| 1997 | Institute of Medicine, USA | 200‒600 Depending on age |
Bones | [145] | ||
| 2010 | Institute of Medicine, USA | 600 to 70 years, 800 for >70 years | 20 | Bones | Based on RCTs | [7] |
| 2011 | Endocrine Society, USA | 1500‒2000 | 30 | Bones, VDD |
Insufficient evidence for non-skeletal | [2] |
| 2013 | International Conference, Experts |
800‒2000; 1600‒4000 for obese |
30-50 | Non-skeletal [143] |
[144] | |
| 2014 | Expert | 4000‒6000 | 40‒52 | Physiological | [146] | |
| 2019 | Experts | 5000‒50,000 | 30‒120 | Treatment, e.g. psoriasis | [147] | |
| 2023 | Experts | Bolus | 30‒50 | Sepsis | [148] | |
| 2024 | Experts | 2000 | 30 | VDD | [149] | |
| 2024 | Endocrine Society, USA | 600‒800 1-18, 75+ years |
VDD | Lack of RCTs, Observational studies ignored |
[1] | |
| 2024 | Experts | 7000‒10,000 | 40‒60 | Obese, multi-morbidity | [150] | |
| 2024 | Experts | 1500‒2000 | 30, 40‒60 preferred |
Skeletal, extra-skeletal | Observational studies | [151] |
| 2024 | Experts | 15‒80 | Disease prevention, treatment (see Figure 6) | Observational studies | [3] |
| Population | Intervention Vitamin D supplementation (IU/d) |
Comparison | Outcome Units ng/mL |
Reference |
|---|---|---|---|---|
| 62 obese (BMI, 37±5 kg/m2, 45±12 year, meant baseline 25(OH)D 20‒26 ng/mL | 1000, 5000, 10,000 for 21 weeks in winter | Dose (IU/day), baseline (ng/mL) 1000 IU, 20±6 5000 IU, 27±7 10,000 IU, 23±15 |
Increments of 25(OH)D 1000 IU, 12±10 5000 IU, 28±10 10,000, 48±20 |
[154] |
| 39 healthy male athletes, 20 years, BMI, 24, UK | 5000 for 14 weeks In winter |
Placebo | 25(OH)D increased from 22 (17‒28) to 50 (39‒60) Vs. 23 (16‒28) to 13 (11‒20) |
[155] |
| 3882 community-based participants, Canada | BMI 22±2 kg/m2 Supplementation (IU/day) Base, 2200, Int, 6100 BMI 27±1 kg/m2 Base, 2100, Int, 6800 BMI 34±4 kg/m2 Base, 1900, Int, 7700 For 6‒18 months |
BMI 22±2 kg/m2 Base, 37 (SD 12), Int, 52 (SD 16) BMI 27±1 kg/m2 Base, 35 (SD 11), Int, 50 (SD 15) BMI 34±4 kg/m2 Base, 32 (SD 10), Int, 47 (SD 15) |
[156] | |
| Long-term hospitalized patients, USA | N = 36, 5000/day, 12 months N = 78, 10,000 IU/day 12 months |
5000 IU, Base 24, Ach, 68 (range, 41‒‒95) 10,000 IU, Base 25, Ach, 96 (range, 53‒‒148) |
[147] | |
| 2423 overweight/ obese (Mean BMI, 32 [SD 4]) prediabetes patients, USA | 4000/day, 24 months | Base, 28 (SD 10) Ach, 54 (SD 15) |
[157] | |
| 30 healthy adults, BMI <30 kg/m2 | 600, 4,000 or 10,000 IU/d of vitamin D3 for 6 months | 162, 320 and 1289 genes up- or down-regulated in their white blood cells, respectively | [24] | |
| 67 T2DM patients with peripheral neuropathy, BMI, 30 (SD 2) kg/m2 Russia | 40,000/week, 24 weeks | 5000/week, 24 weeks | 40,000 IU Base, 16 (SD 8), Ach, 72 (SD 17) 5000 IU Base, 19 (SD 8), Ach, 27 (SD 7) |
[158] |
| 2423 overweight/ obese prediabetes patients, USA | 4000 for three years | Placebo | Achieved 25OHD Adverse events, RR = 0.94 (95%, 0.90‒0.98) |
[159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





