1. Introduction
Aging is a complex process that can be generally defined as the time-dependent decline of physiological functions affecting living organisms. With time, this leads to a decline in immune efficacy, resulting in increased vulnerability to infectious diseases, diminished responses to vaccination, and a susceptibility to age-related inflammatory diseases [
1]. Moreover, Immunosenescence is characterized by a progressive deterioration of the immune system associated with aging. Multiple components of both innate and adaptive immune systems experience aging-related changes, such as alterations in the number of circulating monocytic and dendritic cells, reduced phagocytic activities of neutrophils, limited diversity in B/T cell repertoire, T cell exhaustion or inflation, and chronic production of inflammatory cytokines known as inflammation. Given this extensive panorama associated with immunosenescence processes, contemporary research in aging requires the capture, visualization, analysis, and interpretation of large amounts of highly complex data [
2].
The set of age-related diseases considered in the present study is made up of those with higher incidence and prevalence worldwide such as cancer, cardiovascular diseases, metabolic diseases, and neurodegenerative diseases. Moreover, chronic systemic sterile inflammation, known as inflammaging, is crucially involved with the etiology and progression of these conditions [
3]. The most common metabolic diseases are type 2 diabetes (T2D), obesity, dyslipidemia, hepatic steatosis, and metabolic syndrome. The most common age-related neurodegenerative diseases include Alzheimer’s disease (AD) and Parkinson’s disease (PD). Rheumatoid arthritis (RA) is a chronic inflammatory disease, associated with symmetrical and destructive inflammation in joints and other tissues. Several factors are involved with an age-related increase in carcinogenesis, including the accumulation of senescent cells, inflammaging, and immunosenescence change associated with poor immune surveillance. Finally, cardiovascular diseases are classified as a group of heart and blood vessel disorders, associated with features of organismal aging, and loss of homeostasis, with increased morbidity and mortality rates.
The conceptual analysis of age-related diseases was performed using the theoretical framework of Formal Concept Analysis (FCA), a mathematical theory oriented, in particular, at applications in knowledge representation, knowledge acquisition, and data analysis in many fields of research [
4,
5,
6,
7,
8]. Briefly, knowledge representation incorporates findings from psychology about how humans solve problems and represent knowledge to design formalisms that will make complex systems easier to design and build, it is grounded on an understanding of human thinking based on concepts, which according to the main philosophical tradition, are constituted by its extension, comprising all the objects, in this case, the set of age-related diseases, which belong to the concept; and its intension, the set of immunosenescence markers, which applies to all objects of the extension [
9].
The theoretical approach used seeks to shed light on the answers to the following questions: Is there a hierarchy among age-related diseases given the ubiquitous presence of immunosenescence markers? If this is the case, what does tell us about this structure about the nature of immunosenescence? And, what implications should be considered regarding therapeutic interventions for these diseases? FCA allowed us to establish a hierarchy between diseases related to aging represented by a conceptual lattice, where a collective made up of inflammaging, expansion of CD28- cells, and expansion of M1 macrophages, is located at the top of the hierarchy. In addition, and focused on metabolic diseases, there are two super concepts, the first encompassing cancer and cardiovascular diseases and the second encompassing rheumatoid arthritis and neurodegenerative diseases.
Another result to emphasize is related to the fact of seeing immunosenescence not as a singular phenomenon but as a process with two different facets, the innate immunosenescence and the acquired immunosenescence, which implies different approaches in its treatments, the first from the behavioral, economic, political and social point of view, without doubts the most cost-effective treatments. Second, it must be addressed using high-cost immunotherapies.
2. Knowledge Processing by Formal Concept Analysis
“Conceptual Knowledge Processing” is grounded on an understanding of human thinking based on concepts [
10]. A
concept is constituted by its
extension (extens), comprising all objects which belong to the concept (as the reader belongs to the concept “human being”), and its
intension (intens), including all attributes, properties, or meanings, which apply to all objects of the extension (all the human beings share the attribute “can think”). From a philosophical point of view, concepts can be understood as the basic units of thought formed in dynamic processes with social and cultural environments and can only make sense regarding the relationships with many other concepts where the
subconcept-superconcept-relation plays a prominent role. Being a subconcept of a superconcept means that the extension of the subconcept is contained in the extension of the superconcept which is equivalent to the relationship that the intension of the subconcept contains the intension of the superconcept [
11].
The theory of ordered sets and lattices provides a natural setting in which to discuss and analyze such concept hierarchies and, to formulate a mathematical model about
objects,
attributes, and relationships that indicate that an object has an attribute, the notion of
formal context was introduced by R. Wille in 1982 [
12], leading to the mathematical theory of Formal Concept Analysis (FCA).
Adapted for the matter of the present study, a formal context is defined as a set structure , where D corresponds to the age-related diseases, in the present case, Neurodegenerative diseases, Rheumatoid arthritis, cancer, Cardiovascular diseases, and metabolic diseases. M is the set of attributes (or markers) of immunosenescence commonly observed in age-related diseases, and finally, the letter I represents the relationship “the object d has the attribute m”, thus
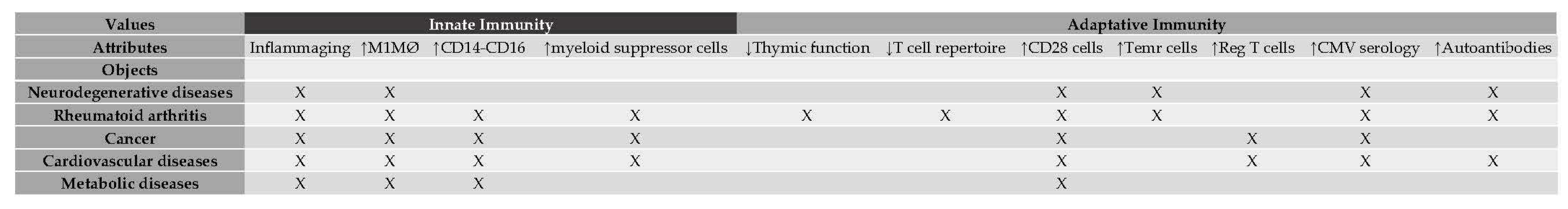
It is important to note that usually is difficult to list all the objects belonging to a concept, and practically impossible to list all its attributes, it is natural to work within a specific context in which the objects and attributes are fixed. The list of objects and their attributes employed in the present study is presented in
Table 1 and were taken from Barbé-Tuana, Funchal, Schmitz et. al. [
3] and other authors [
13,
14,
15].
Given that immunosenescence has defined the destruction and remodeling of immune organ structure as well as innate and adaptive immune dysfunction with aging, a bi-valued formal context is constructed highlighting those attributes (immunosenescence features/markers) related to innate immune processes and those related to acquired immunity. A formal context is best understood if it is depicted by a cross table as follows: that the
ith object possesses the
jth attribute indicated by an X in the
ij position of the table (
Table 2).
From the formal context
, consider
and
, define
so,
A’ is the set of immunosenescence markers common to all age-related diseases in
A and B’ is the set of age-related diseases possessing the markers in B.
The Concept of the context (
D, M, I) is defined to be a pair (
A, B) where
and
,
A’= B and
B’=A. The set of all concepts of the context
is denoted
ẞ (D, M, I) and the Fundamental Theorem of FCA demonstrates that
ẞ (D, M, I) is a complete lattice, usually denoted
and named the
Concept Lattice of the context
K. Fortunately, there are several simple and fast algorithms for generating formal concepts and for constructing and navigating concept lattices. In the present study, the software Concept Explorer-CONEXP was employed [
16,
17].
3. Results
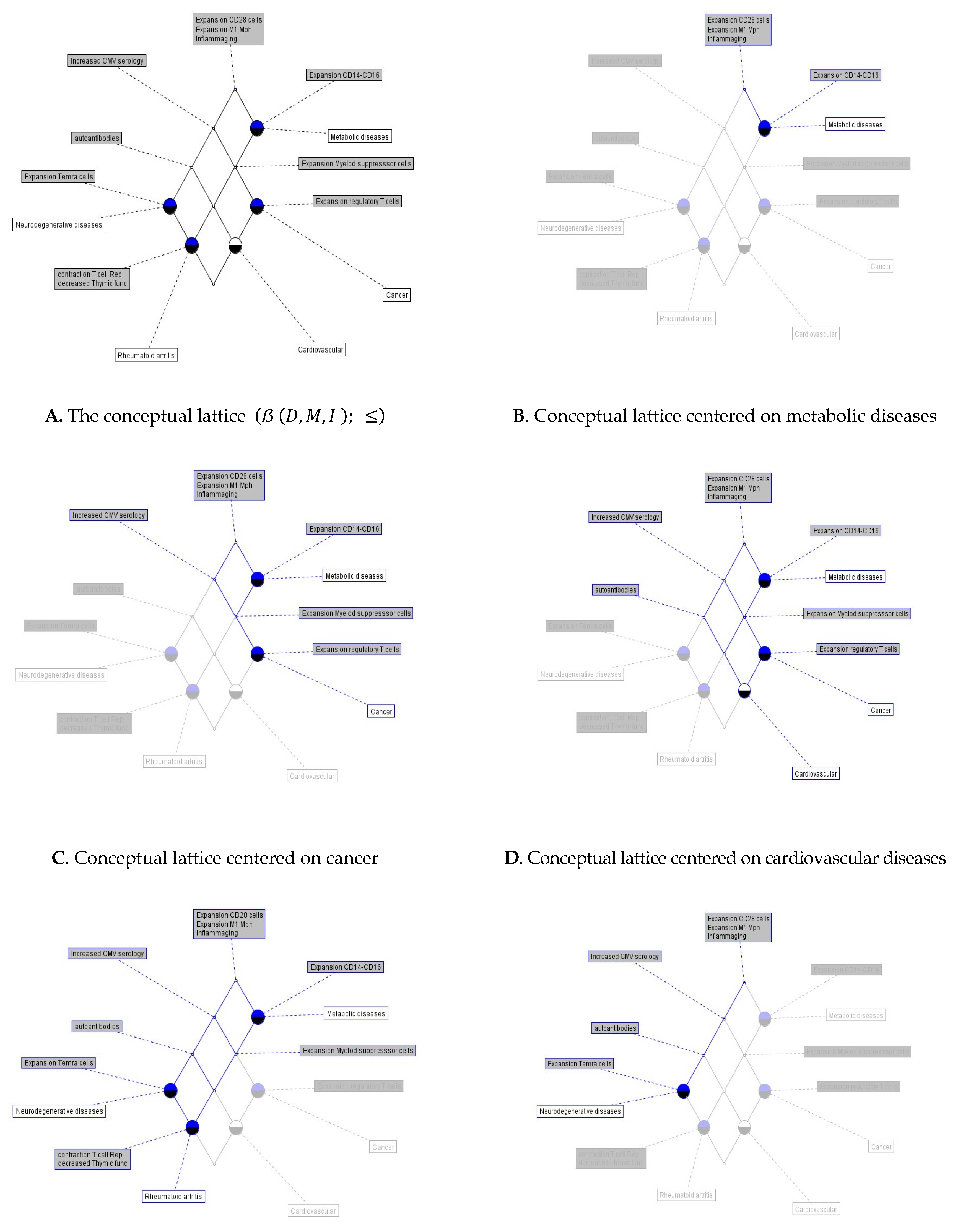
Once the formal context is defined its corresponding concept lattice is derived, which is best pictured by a labeled line diagram like those in
Figure 1 numerals A to F, where the concept lattice of age-related diseases and its associated immunosenescence markers
is observed in
Figure 1A, where the
Sup of the lattice is a set of immunosenescence markers whose elements are {inflammaging, expansion of CD28
- cells, expansion of M1 macrophages}which are shared by all the age-related diseases considered in the present study. Note that this set consists of markers associated principally with the innate immune compartment, and given that these markers are related between them, we denote them as
the inflammaging collective. This
collective command a hierarchical structure of age-related disease concepts, represented in the lattice by circles.
It is possible to read off the context from the lattice by looking at the extension belonging to one of the circles representing the concepts and following a descending line path from the selected upper circle. To read off the concept lattice and its extens and intens, we set off each circle following a clockwise descending line from the collective. In
Figure 1B, the
object-subconcept {Metabolic diseases} is associated with the collective plus the expansion of CD14
++ and CD16
+ cells, indicating that metabolic diseases such as type 2 diabetes (T2D), obesity, dyslipidemia, hepatic steatosis, and metabolic syndrome, actually dramatically increasing worldwide, are related with processes that mainly involve mechanisms of innate immunity. In
Figure 1C setting off the circle (Cancer), we obtain the
object-concept {Metabolic diseases, Cancer} with newly added immunosenescence markers mainly involving mechanisms of acquired immunity like {Increased CMV serology, Expansion of myeloid-derived suppressor cells, expansion of regulatory T cells} indicating the well-documented association between metabolic diseases and cancer, but also, indicating which immunosenescence markers linked with acquired immunity can be involved.
Setting off (Cardiovascular) we obtain the
object-superconcept {Metabolic diseases, Cancer, Cardiovascular diseases} with those immunosenescence markers already present in the previous object-concept plus the presence of {Autoantibodies} (
Figure 1D). Thus, regarding immunosenescence markers, these three diseases seem to be closely related. Setting off (Rheumatoid arthritis) another
object-superconcept (
Figure 1E) is obtained {Metabolic diseases, Rheumatoid arthritis, Neurodegenerative diseases} with immunosenescence markers linked with innate immunity, those of metabolic diseases, plus several markers linked with acquired immunity {Increased CMV serology, Expansion of myeloid suppressor cells, Autoantibodies, Expansion of T
EMRA cells, Contraction of T cell repertoire, Decreased thymic function} indicating a very complex network of immune mechanisms, mainly the acquired nature, underlying these three age-related diseases.
Finally, when we set off (Neurodegenerative diseases) an
object-subconcept (
Figure 1F) appears {Neurodegenerative diseases} without linkages with other age-related diseases but with the attribute set {the
Collective, Increased CMV serology, Autoantibodies, Expansion of T
EMRA cells} suggesting that neurodegenerative diseases like Alzheimer and Parkinson, could be linked exclusively with innate and acquired immunosenescence processes without the concurrent action of other age-related diseases.
4. Discussion
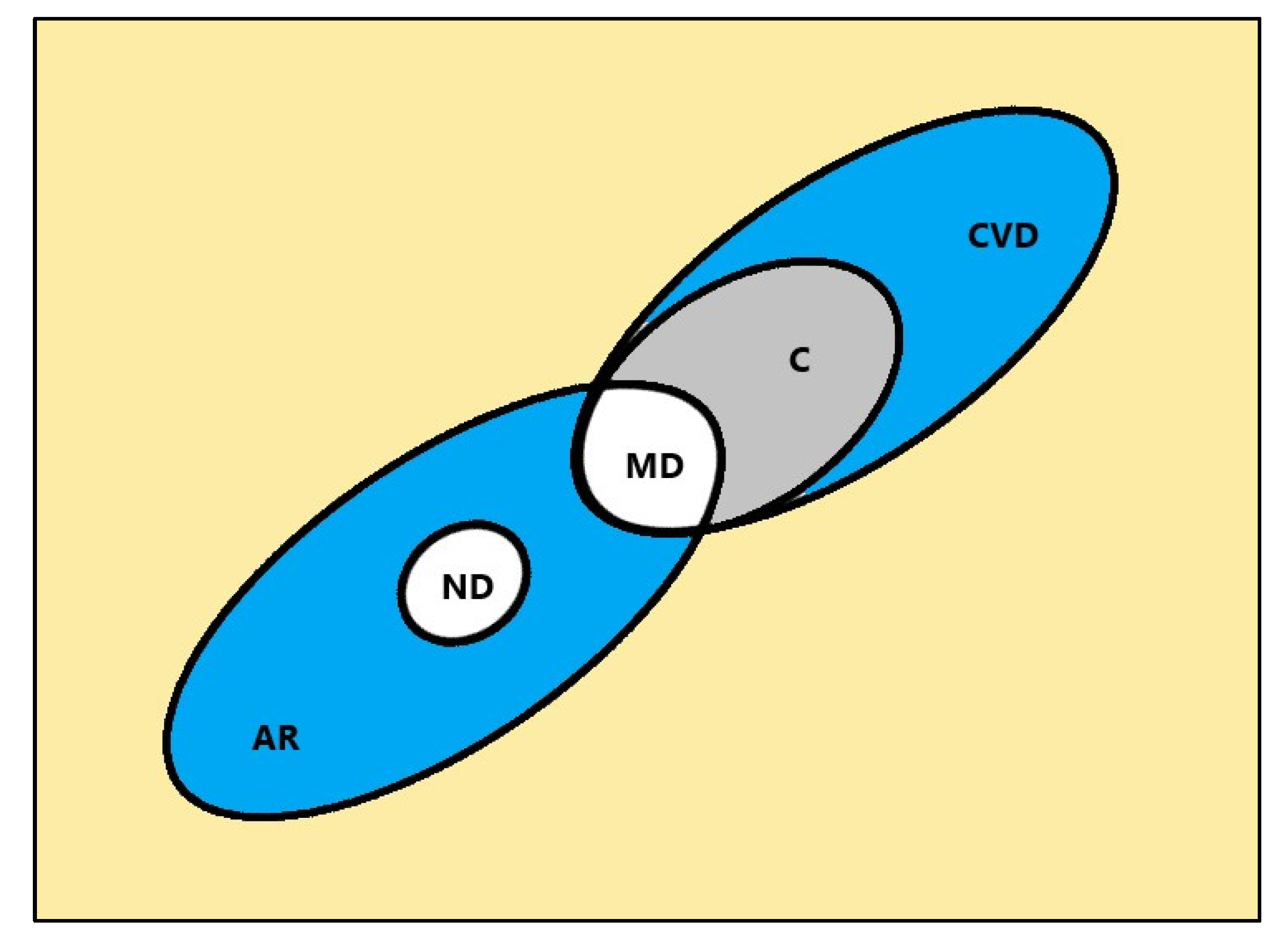
Senescence is considered a universal process that afflicts all lifeforms on earth, and of course, all human beings, but celerity and expression level of its manifestations depends on habits, environment, and culture within which the individual is immersed. Clinical research in age-related diseases like cancer, cardiovascular ailments, and metabolic and neurodegenerative diseases among others, has identified immunosenescence as a key process in the dynamic of senescence in humans and many other species. Knowledge processing by FCA of age-related diseases framed in an immunosenescence multivalued context indicates that a hierarchy among these diseases emerges with a subconcept-superconcept nested structure like those illustrated with a Venn diagram in
Figure 2.
Another implication of the ontological analysis of age-related diseases is about the nature of immunosenescence, indicating that initial symptoms of age-related diseases are mainly related to innate immune mechanisms, but the development of severe symptoms of age-related diseases like cancer, cardiovascular or neurodegenerative diseases depends on the activation of acquired immunity processes. This way, it is possible to suggest a “Janus face” of immunosenescence, innate derived immunosenescence, and acquired derived immunosenescence, not just for its causal events but also, because the treatments are truly different depending on which compartment the patient is located (
Table 3), the former mainly with changes in social and cultural habits and customs, and the later with immune network operations.
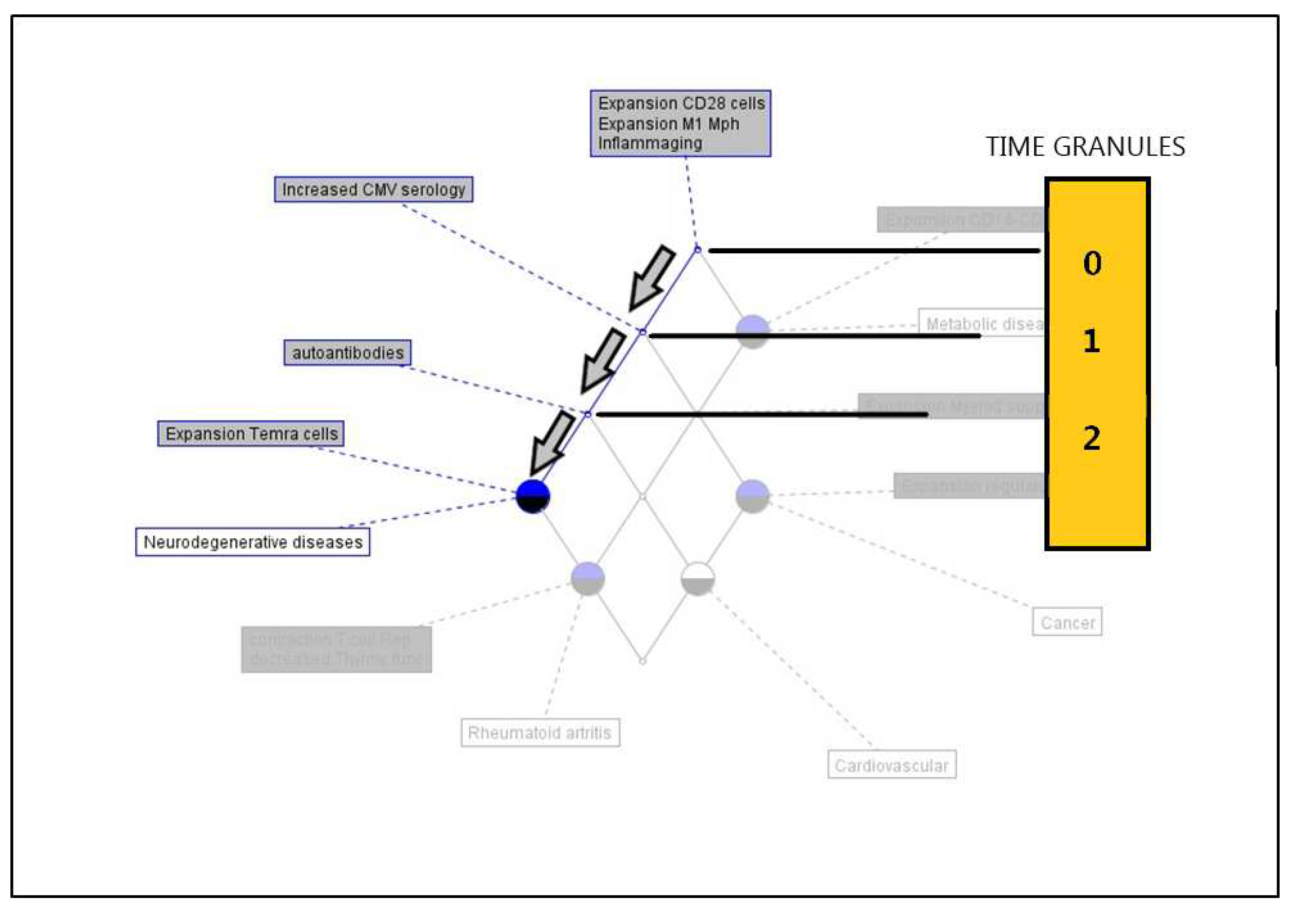
A limitation of the knowledge representation as a model of the hierarchy of age-related diseases, in the framework of immunosenescence markers, is related to the absence of the temporal dimension, so although we can derive a consistent hierarchy between the age-related diseases considered, it is not possible to establish a natural timeline between the occurrence of the immunosenescence markers and its association with these diseases. To solve this flaw, we can use the notion of
Conceptual Time Systems [
19].
In brief, what is
time in a conceptual time system? Let us imagine that we observe a real system. For a single observation, we need some time to be a minute or an hour. Often, we abstract from the duration of an observation and use the notion of a point in time, usually represented by a real number. Following this idea, it is possible to define a set
T whose elements are called time granules. For describing the observations, for instance, the clinical detection of some innate or acquired immunosenescence marker, we introduce a new many-valued context with
T as a set of formal objects. In a data table of this many-valued context, the row time granules show a column for the measurement of a given marker at time granule
t. As is shown in
Figure 3, for example, in neurodegenerative diseases at time t = 0, the
collective of immunosenescence markers is present.
Age-related diseases are the major challenge faced by public health and they still need of robust preventive measures and disease-modifying treatments. Population-based studies can offer the framework in the context of primary and secondary prevention of age-related diseases. The epidemiology of age-related disorders in the last decades has focused on descriptive studies mainly based on the use of clinical criteria. However, clinical definition is insufficient both to well-characterize different phenotypes and to make an early diagnosis. Descriptive epidemiology needs a new framework from health data science, like knowledge representation, to update the area of research on age-related diseases, based on the advancement of both clinical and biological diagnostic criteria and the urgency for an early diagnosis of these diseases.
Funding
This work was supported by Fundación Universitaria San Mateo.
Data Availability Statement
Data is freely available upon request
Conflicts of Interest
The author declares that there is no conflict of interest regarding the publication of this article.
References
- Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014 Jan;30(1):16-22. [CrossRef] [PubMed]
- Romanyukha, A. A., Rudnev, S. G., Sannikova, T. A., & Yashin, A. I. (2009). Mathematical modeling of immunosenescence: Scenarios, processes and limitations. In Handbook on Immunosenescence: Basic Understanding and Clinical Applications (Vol. 9781402090639, pp. 145–163). [CrossRef]
- Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Semin Immunopathol. 2020 Oct;42(5):545-557. Epub 2020 Aug 3. PMCID: PMC7398288. [CrossRef] [PubMed]
- Bernhard Ganter, Rudolf Wille, and C. Franzke. 1997. Formal Concept Analysis: Mathematical Foundations (1st. ed.). Springer-Verlag, Berlin, Heidelberg.
- Burgos-Salcedo Javier (2021) A rational strategy to support approved COVID-19 vaccines prioritization, Human Vaccines & Immunotherapeutics, 17:10, 3474-3477. [CrossRef]
- Burgos-Salcedo J. 2021. A comparative analysis of clinical stage 3 COVID-19 vaccines using knowledge representation. MedRxiv, Cold Spring Harbor Laboratory Press.
- Rubén D. Bourdon-García and Javier D. Burgos-Salcedo 2016 J. Phys.: Conf. Ser. 738 012054.
- Alonso Restrepo de León, Hugo Sotomayor, Carolina Sierra, Javier Burgos-Salcedo. A Conceptual Analysis of Ancient Ecuadorian Faces Carved in Spondylus Shells. Sch J Eng Tech, 2022 Apr 10(4): 54-60.
- Wille, R. (2008). Formal Concept Analysis as Applied Lattice Theory. In: Yahia, S.B., Nguifo, E.M., Belohlavek, R. (eds) Concept Lattices and Their Applications. CLA 2006. Lecture Notes in Computer Science (eds), vol 4923. Springer, Berlin, Heidelberg. [CrossRef]
- Wille, R. (2005). Formal Concept Analysis as Mathematical Theory of Concepts and Concept Hierarchies. In: Ganter, B., Stumme, G., Wille, R. (eds) Formal Concept Analysis. Lecture Notes in Computer Science(eds), vol 3626. Springer, Berlin, Heidelberg. [CrossRef]
- Wille, R. (2005). Conceptual Knowledge Processing in the Field of Economics. In: Ganter, B., Stumme, G., Wille, R. (eds) Formal Concept Analysis. Lecture Notes in Computer Science (eds), vol 3626. Springer, Berlin, Heidelberg. [CrossRef]
- Wille, R. (1982). Restructuring Lattice Theory: An Approach Based on Hierarchies of Concepts. In: Rival, I. (eds) Ordered Sets. NATO Advanced Study Institutes Series, vol 83. Springer, Dordrecht. [CrossRef]
- Pawelec G. Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing. (2012) 9:15. [CrossRef]
- Oh SJ, Lee JK, Shin OS. Aging and the Immune System: the Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity. Immune Netw. 2019 Nov 14;19(6): e37. PMCID: PMC6943173. [CrossRef] [PubMed]
- Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol. 2019 Sep 25; 10:2247. PMID: 31608061; PMCID: PMC6773825. [CrossRef]
- Serhiy A. Yevtushenko. System of data analysis “Concept Explorer”. (In Russian). Proceedings of the 7th national conference on Artificial Intelligence KII-2000, p. 127-134, Russia, 2000.
- https://conexp.sourceforge.net/index.html.
- Burgos-Salcedo J. Immune network operations in COVID-19. Explor Immunol. 2022; 2:572–80. [CrossRef]
- Wolff, K.E. (2005). States, Transitions, and Life Tracks in Temporal Concept Analysis. In: Ganter, B., Stumme, G., Wille, R. (eds) Formal Concept Analysis. Lecture Notes in Computer Science (eds), vol 3626. Springer, Berlin, Heidelberg. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).