Submitted:
14 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- influenced the extent of conjugation;
- affected dipole moment;
- changed backbone rigidity,
- brought new functionalities such as strong stacking between the aromatic units,
- altered the system’s response to light stimulus (i.e. accelerated collective switching behavior or caused significant broadening of the optical absorption spectra);
- emphasized the role of spacer length [11]: a shorter spacer should facilitate the charge transfer rate through the junction and increase conductance, while a longer spacer is prone to decoupling of the Azo from the electrode, therefore
- allowing larger dynamics of the switching event, and consequently
- a larger ON/OFF conductance ratio.
2. Models and Methods
3. Results
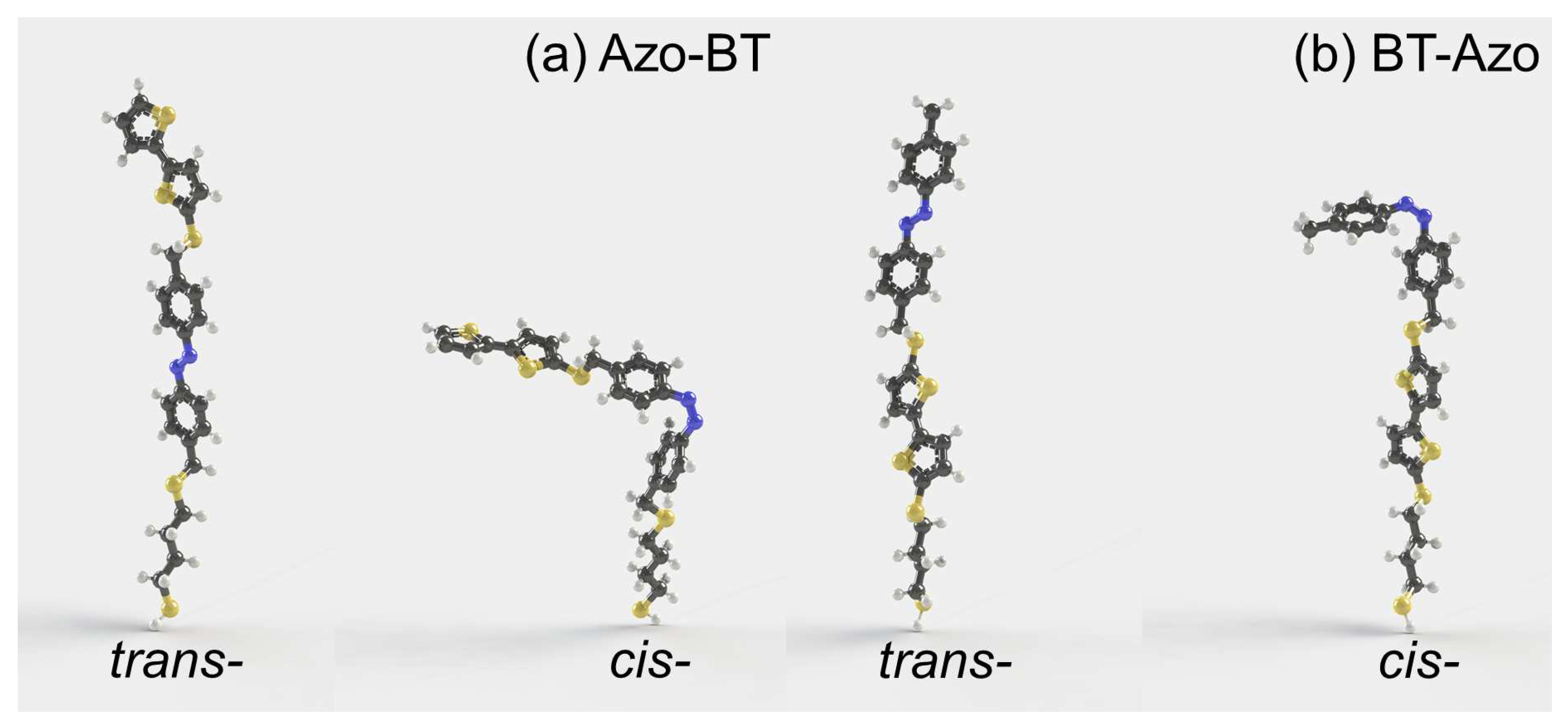
3.1. Properties of isolated molecules
- the values of dipole moments (1.34 and 3.22 D for trans- and cis-BT-Azo, 1.67 and 3.52D for trans- and cis-Azo-BT);
- the differences in the length of trans- and cis-isomers h is 6.05Å and 14.69Å for BT-Azo and Azo-BT, respectively; the experimental value of h=5.1Å for the layer of BT-Azo [23];
- even though the molecular volume reduces upon trans-cis isomerization for both MS, the intermolecular sterical clashes may arise for cis-populated layers, especially for Azo-BT MS.
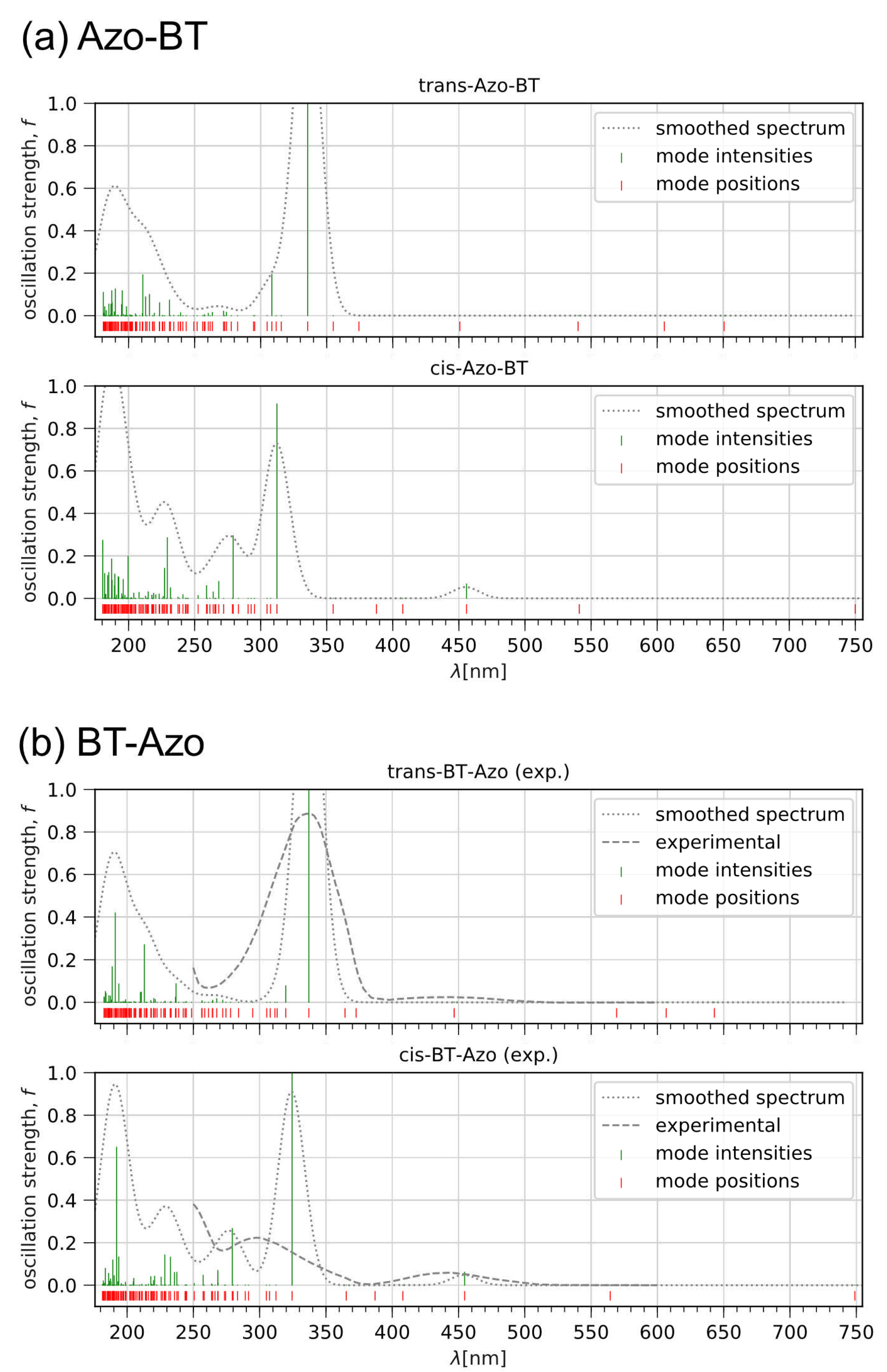
3.1.1. Optical properties
3.1.2. Reorganization energies
3.1.3. Gibbs free energy of solvation and its changes upon light stimulus
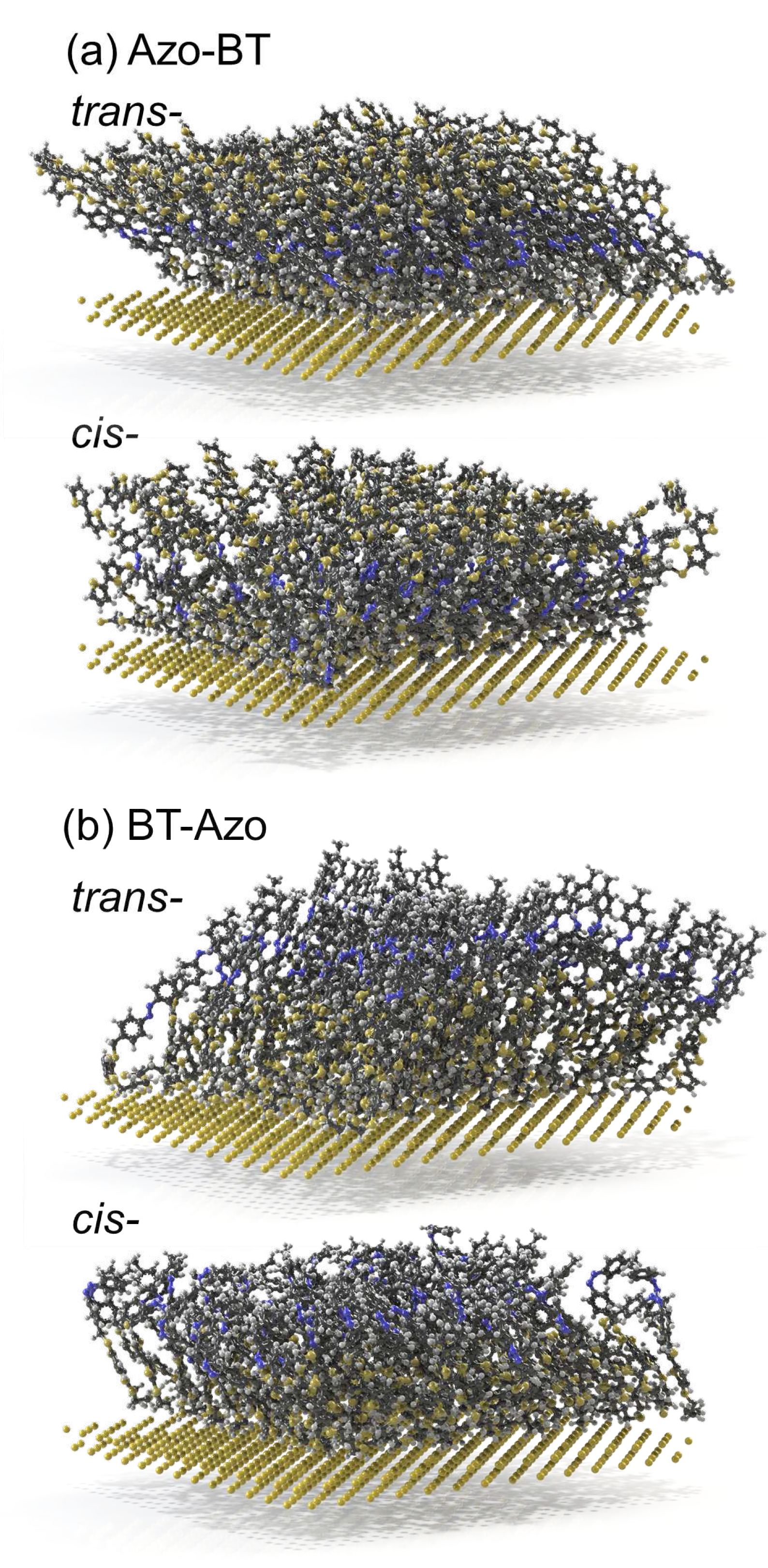
3.2. Properties of chemisorbed monolayers
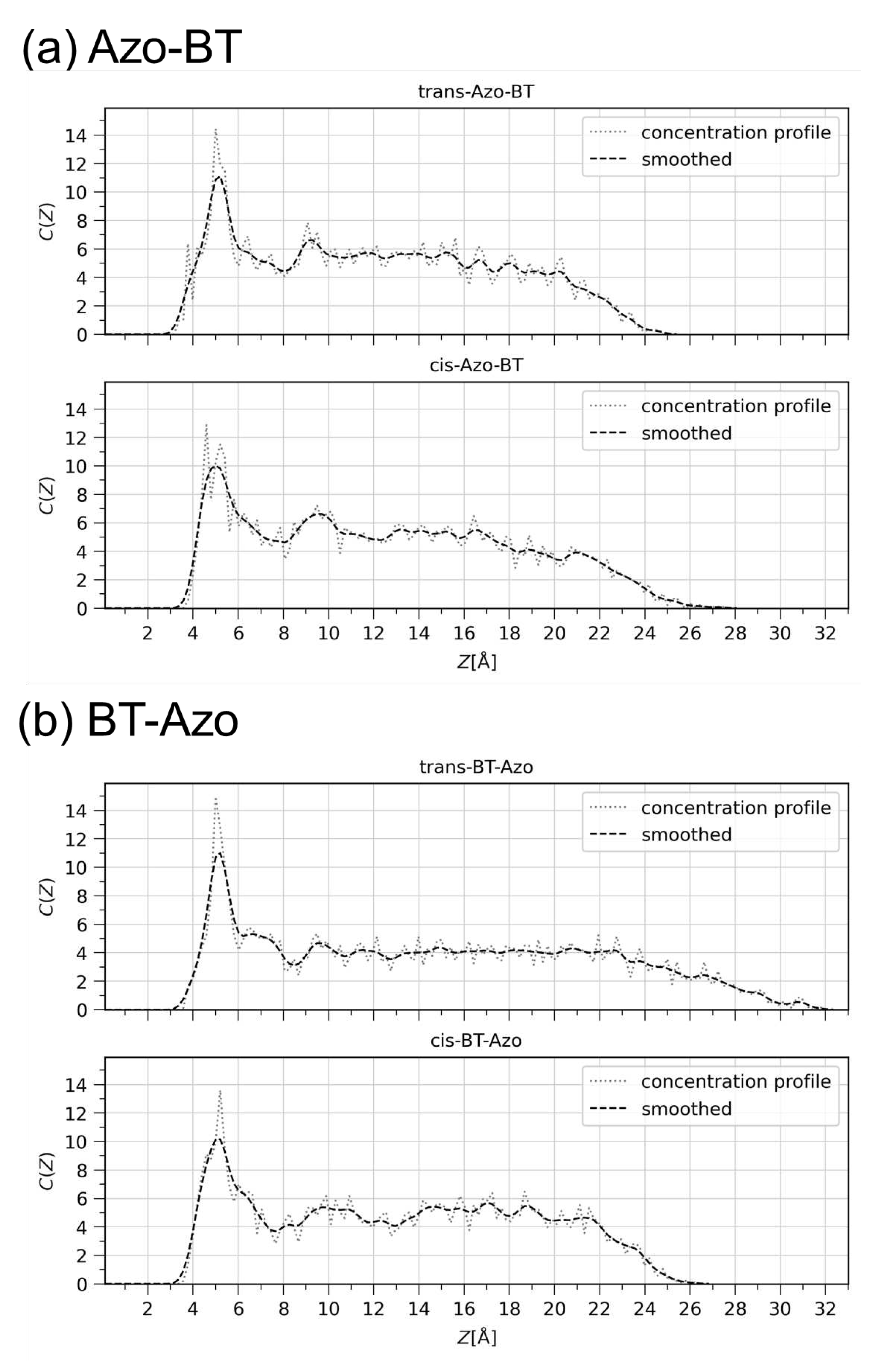
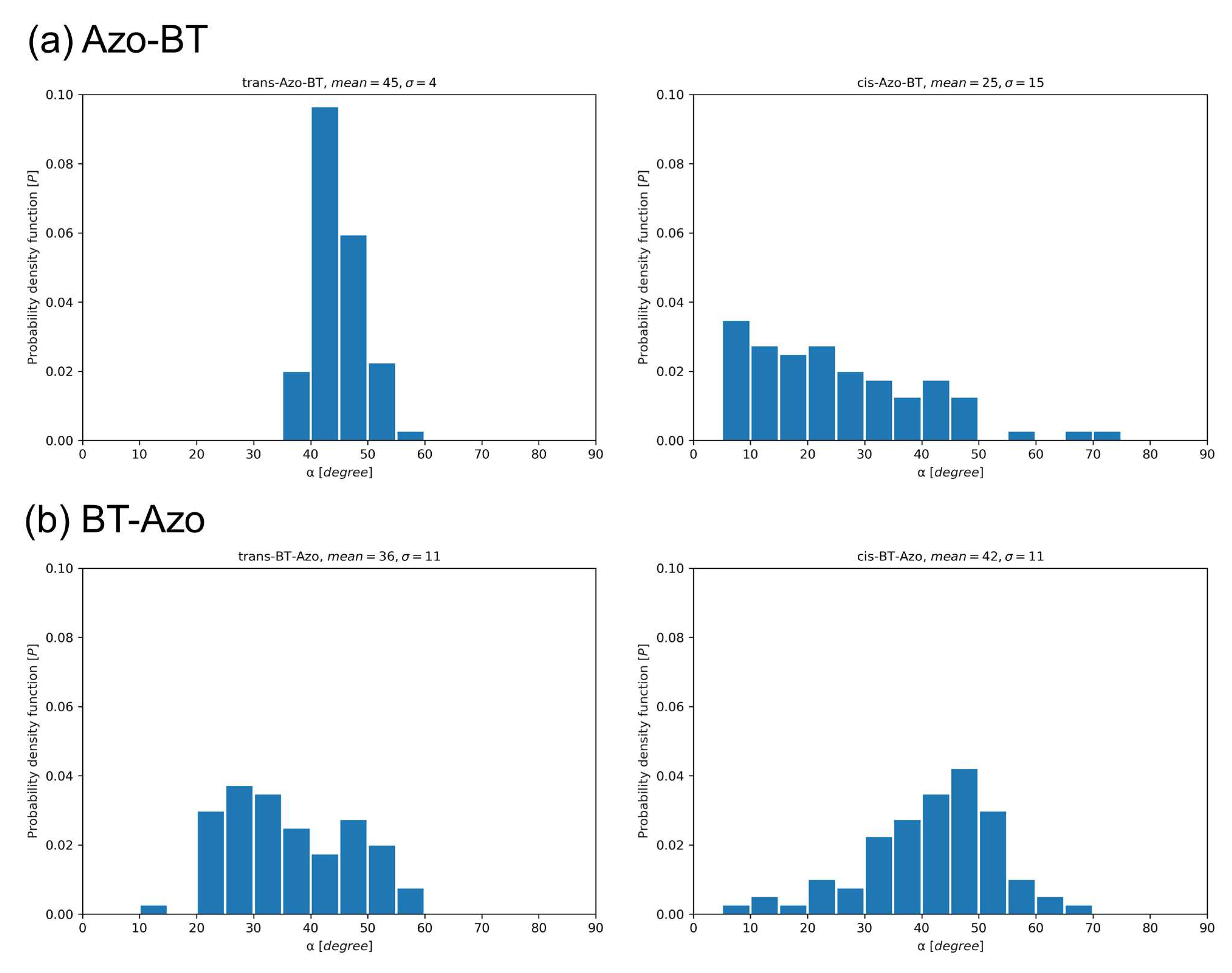
3.2.1. Structural properties
3.2.2. Photo-programmable charge transfer
4. Discussion and conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Azo | Azobenzene |
| BT | Bithiophene |
| MS | Molecular switch |
| MJ | Molecular junction |
| DFT | Density functional theory |
| MD | Molecular dynamics simulation |
References
- Fuentes, N., Martin-Lasanta, A., de Cienfuegos, L. Á., Ribagorda, M., Parra, A., Cuerva, J. M. Organic-based molecular switches for molecular electronics. Nanoscale 2011, 3, 4003–4014. [CrossRef]
- Klajn, R. Immobilized azobenzenes for the construction of photoresponsive materials. Pure Appl. Chem. 2010, 82, 2247–2279. [Google Scholar] [CrossRef]
- Delorme, N., Bardeau, J.-F., Bulou, A., Poncin-Epaillard, F. Azobenzene-containing monolayer with photoswitchable wettability. Langmuir 2005, 21, 12278–12282.
- Takahashi, I., Honda, Y., Hirota, S. Regulating copper-binding affinity with photoisomerizable azobenzene ligand by construction of a self-assembled monolayer. Angew. Chem. Int. Ed. 2009, 48, 6065–6068. [CrossRef]
- Callari, F. L., Petralia, S., Conoci, S., Sortino, S. Light-triggered DNA release by dynamic monolayer films. New J. Chem. 2008, 32, 1899–1903. [CrossRef]
- Huang, X., Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [CrossRef]
- Liu, Y., Qiu, X., Soni, S., Chiechi, R. C. Charge transport through molecular ensembles: Recent progress in molecular electronics. Chem. Phys. Rev. 2021, 2, 021303. [CrossRef]
- Tang, C., Shiri, M., Zhang, H., Ayinla, R. T., Wang, K. Light-driven charge transport and optical sensing in molecular junctions. Nanomaterials 2022, 12, 698. [CrossRef]
- Yu, S. H., Hassan, S. Z., So, C., Kang, M., Chung, D. S. Molecular-switch-embedded Solution-processed Semiconductors. Adv. Mater. 2022, 35, 2203401. [CrossRef]
- Karpe, S., Oçafrain, M., Smaali, K., Lenfant, S., Vuillaume, D., Blanchard, P., Roncali, J. Oligothiophene-derivatized azobenzene as immobilized photoswitchable conjugated systems. Chem. Commun. 2010, 46, 3657–3659. [CrossRef]
- Smaali, K., Lenfant, S., Karpe, S., Oçafrain, M., Blanchard, P., Deresmes, D., Godey, S., Rochefort, A., Roncali, J., Vuillaume, D. High ON-OFF conductance switching ratio in optically-driven self-assembled conjugated molecular systems. ACS Nano 2010, 4, 2411–2421. [CrossRef]
- Ferri, V., Elbing, M., Pace, G., Dickey, M. D., Zharnikov, M., Samorì, P., Mayor, M., Rampi, M. A. Light-powered electrical switch based on cargo-lifting azobenzene monolayers. Angew. Chem. Int. Ed. 2008, 18, 3455–3457. [CrossRef]
- Schuster, S., Füser, M., Asyuda, A., Cyganik, P., Terfort, A., Zharnikov, M. Photoisomerization of azobenzene-substituted alkanethiolates on Au (111) substrates in the context of work function variation: the effect of structure and packing density. Phys. Chem. Chem. Phys. 2019, 21, 9098–9105. [CrossRef]
- Mativetsky, J. M., Pace, G., Elbing, M., Rampi, M. A., Mayor, M., Samori, P. Azobenzenes as light-controlled molecular electronic switches in nanoscale metal- molecule- metal junctions. J. Am. Chem. Soc. 2008, 130, 9192–9193. [CrossRef]
- Thomas, L., Arbouch, I., Guérin, D., Wallart, X., Van Dyck, C., Mélin, T., Cornil, J., Vuillaume, D., Lenfant, S. Conductance switching of azobenzene-based self-assembled monolayers on cobalt probed by UHV conductive-AFM. Nanoscale 2021, 13, 6977–6990. [CrossRef]
- Crivillers, N., Liscio, A., Di Stasio, F., Van Dyck, C., Osella, S., Cornil, D., Mian, S., Lazzerini, G.M.., Fenwick, O., Orgiu, E. and others. Photoinduced work function changes by isomerization of a densely packed azobenzene-based SAM on Au: a joint experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 14302–14310. [CrossRef]
- Masillamani, A. M., Osella, S., Liscio, A., Fenwick, O., Reinders, F., Mayor, M., Palermo, V., Cornil, J., Samorì, P. Light-induced reversible modification of the work function of a new perfluorinated biphenyl azobenzene chemisorbed on Au (111). Nanoscale 2014, 6, 8969–8977. [CrossRef]
- Yu, S. H., Hassan, S. Z., Nam, G.-H., An, S., Kang, B., Chung, D. S. Consideration of azobenzene-based self-assembled monolayer deposition conditions for maximizing optoelectronic switching performances. Chem. Mater. 2021, 33, 5991–6002. [CrossRef]
- Ah Q., Lloyd F.N., Akiyama, H., Nagahiro, T., Tamada, K., Wee, A.T.S. Reversible work function changes induced by photoisomerization of asymmetric azobenzene dithiol self-assembled monolayers on gold. Appl. Phys. Lett. 2008, 93, 083109. [CrossRef]
- Yamamoto, T., Natsui, K., Einaga, Y. Photo-Modulation of Superconducting and Magnetic Properties. In: Photon-Working Switches; Springer, 2017; pp. 285–299.
- Suda, M., Kameyama, N., Ikegami, A., Suzuki, M., Kawamura, N., Einaga, Y. Size-reduction induced ferromagnetism and photo-magnetic effects in azobenzene-thiol-passivated gold nanoparticles. Polyhedron 2009, 28, 1868–1874. [CrossRef]
- Karthäuser, S., Peter, S., Simon, U. Integration of individual functionalized gold nanoparticles into nanoelectrode configurations: Recent advances. Eur. J. Inorg. Chem. 2020, 40, 3798–3810. [CrossRef]
- Viero, Y., Copie, G., Guerin, D., Krzeminski, C., Vuillaume, D., Lenfant, S., Cleri, F. High conductance ratio in molecular optical switching of functionalized nanoparticle self-assembled nanodevices. J. Phys. Chem. C 2015, 119, 21173–21183. [CrossRef]
- Ahonen, P., Laaksonen, T., Schiffrin, D. J., Kontturi, K. Photoswitching electron transport properties of an azobenzene containing thiol-SAM. Phys. Chem. Chem. Phys. 2007, 9, 4898–4901. [CrossRef]
- Cho, D., Yang, M., Shin, N., Hong, S. Mapping reversible photoswitching of molecular resistance fluctuations during the conformational transformation of azobenzene-terminated molecular switches. Nanotechnology 2018, 29, 365704. [CrossRef]
- Kumar, A. S., Ye, T., Takami, T., Yu, B.-C., Flatt, A.K., Tour, J. M., Weiss, P. S. Reversible photo-switching of single azobenzene molecules in controlled nanoscale environments. Nano Lett. 2008, 8, 1644–1648. [CrossRef]
- Del Valle, M., Gutiérrez, R., Tejedor, C., Cuniberti, G. Tuning the conductance of a molecular switch. Nature Nanotechnol. 2007, 2, 176–179. [CrossRef]
- Koch, M., Saphiannikova, M., Santer, S., Guskova, O. Photoisomers of azobenzene star with a flat core: Theoretical insights into multiple states from DFT and MD perspective. J. Phys. Chem. B 2017, 121, 8854–8867. [CrossRef]
- Montagna, M., Guskova, O. Photosensitive cationic azobenzene surfactants: Thermodynamics of hydration and the complex formation with poly (methacrylic acid). Langmuir 2018, 34, 311–321. [CrossRef]
- Savchenko, V., Guskova, O. Molecular switch based on bithiophene-azobenzene: How to control conductance through the monolayer using light. Herald of Tver State University, Series: Chemistry 2021, 3, 7–20. [CrossRef]
- Kaneta, M., Honda, T., Onda, K., Han, M. Repeated photoswitching performance of azobenzenes adsorbed on gold surfaces: a balance between space, intermolecular interactions, and phase separation. New J. Chem. 2017, 41, 1827–1833. [CrossRef]
- Tian, Z., Wen, J., Ma, J. Dynamic simulations of stimuli-responsive switching of azobenzene derivatives in self-assembled monolayers: reactive rotation potential and switching functions. Mol. Simul. 2015, 41, 28–42. [CrossRef]
- Cantatore, V., Granucci, G., Rousseau, G., Padula, G., Persico, M. Photoisomerization of self-assembled monolayers of azobiphenyls: Simulations highlight the role of packing and defects. J. Phys. Chem. Lett. 2016, 7, 4027–4031. [CrossRef]
- Wen, J., Tian, Z., Ma, J. Light-and electric-field-induced switching of thiolated azobenzene self-assembled monolayer. J. Phys. Chem. C 2013, 117, 19934–19944. [CrossRef]
- Liu, C., Zheng, D., Hu, W., Zhu, Q., Tian, Z., Zhao, J., Zhu, Y., Ma, J. Tuning the collective switching behavior of azobenzene/Au hybrid materials: flexible versus rigid azobenzene backbones and Au (111) surfaces versus curved Au nanoparticles. Nanoscale 2017, 9, 16700–16710. [CrossRef]
- Fast, E., Schlimm, A., Lautenschläger, I., Clausen, K. U., Strunskus, T., Spormann, C., Lindhorst, T. K., Tuczek, F. Improving the switching capacity of glyco-self-assembled monolayers on Au (111). Chem. Eur. J. 2020, 26, 485–501. [CrossRef]
- Rashid, M. A. M., Hayati, D., Kwak, K., Hong, J. Theoretical investigation of azobenzene-based photochromic dyes for dye-sensitized solar cells. Nanomaterials 2020, 10, 914. [CrossRef]
- Crivillers, N., Orgiu, E., Reinders, F., Mayor, M., Samorì, P. Optical modulation of the charge injection in an organic field-effect transistor based on photochromic self-assembled-monolayer-functionalized electrodes. Adv. Mat. 2011, 23, 1447–1452. [CrossRef]
- Tirosh, E., Benassi, E., Pipolo, S., Mayor, M., Valášek, M., Frydman, V., Corni, S., Cohen, S. R. Direct monitoring of opto-mechanical switching of self-assembled monolayer films containing the azobenzene group. Beilstein J. Nanotechnol. 2011, 2, 834–844. [CrossRef]
- Crivillers, N., Osella, S., Van Dyck, C., Lazzerini, G. M., Cornil, D., Liscio, A., Di Stasio, F., Mian, S., Fenwick, O., Reinders, F., Neuburger, M, Treossi, E., Mayor, M., Palermo, V., Cacialli, F., Cornil, J., Samorì, P. Large work function shift of gold induced by a novel perfluorinated azobenzene-based self-assembled monolayer. Adv. Mat. 2013, 25, 432–436. [CrossRef]
- Osella, S., Samori, P., Cornil, J. Photoswitching azobenzene derivatives in single molecule junctions: A theoretical insight into the I/V characteristics. J. Phys. Chem. C 2014, 118, 18721–18729. [CrossRef]
- Van Dyck, C., Bergren, A. J., Mukundan, V., Fereiro, J. A., DiLabio, G. A. Extent of conjugation in diazonium-derived layers in molecular junction devices determined by experiment and modelling. Phys. Chem. Chem. Phys. 2019, 21, 16762–16770. [CrossRef]
- Rego, L. G. C., Bortolini, G. Modulating the photoisomerization mechanism of semiconductor-bound azobenzene-functionalized compounds. J. Phys. Chem. C 2019, 123, 5692–5698. [CrossRef]
- Bang, G. S., Lee, J., Baek, H. Y., Lee, H., Jun, K., Shin, S. R. Adsorption of azothiophene dye having an n-bridging bidentate tail group on gold. Langmuir 2009, 25, 10788–10793. [CrossRef]
- Viero, Y., Guérin, D., Vladyka, A., Alibart, F., Lenfant, S., Calame, M., Vuillaume, D. Light-stimulatable molecules/nanoparticles networks for switchable logical functions and reservoir computing. Adv. Func. Mater. 2018, 28, 1801506. [CrossRef]
- Stiévenard, D., Guérin, D., Lenfant, S., Lévêque, G., Nijhuis, C. A., Vuillaume, D. Electrical detection of plasmon-induced isomerization in molecule–nanoparticle network devices. Nanoscale 2018, 10, 23122–23130. [CrossRef]
- Lenfant, S. Charge transport in dynamic molecular junctions. In: Molecular electronics: An experimental and theoretical approach; CRC Press, 2016; pp. 65–99. [CrossRef]
- Guskova, O. A. On the inter-ring torsion potential of 2, 2’-bithiophene: A review of open problems and current proposals. In: Quantum systems in physics, chemistry, and biology: Advances in concepts and applications; Springer, 2017; pp. 209–230.
- Mennucci, B., Cances, E., Tomasi, J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: theoretical bases, computational implementation, and numerical applications. J. Phys. Chem. B 1997, 101, 10506–10517.
- Malyar, I. V., Titov, E., Lomadze, N., Saalfrank, P., Santer, S. Photoswitching of azobenzene-containing self-assembled monolayers as a tool for control over silicon surface electronic properties. J. Chem. Phys. 2017, 146, 104703. [CrossRef]
- Pipolo, S., Benassi, E., Corni, S. Structural properties of azobenzene self-assembled monolayers by atomistic simulations. Langmuir 2017, 29, 10505–10512. [CrossRef]
- Malagoli, M., Brédas, J.-L. Density functional theory study of the geometric structure and energetics of triphenylamine-based hole-transporting molecules. Chem. Phys. Lett. 2000, 327, 13–17. [CrossRef]
- Nelsen, S. F., Blackstock, S. C., Kim, Y. Estimation of inner shell Marcus terms for amino nitrogen compounds by molecular orbital calculations. J. Am. Chem. Soc. 1987, 109, 677–682. [CrossRef]
- Koh, S. E., Risko, C., da Silva Filho, D. A., Kwon, O., Facchetti, A., Brédas, J.-L., Marks, T. J., Ratner, M. A. Modeling electron and hole transport in fluoroarene-oligothiopene semiconductors: Investigation of geometric and electronic structure properties. Adv. Func. Mater. 2008, 18, 332–340. [CrossRef]
- Savchenko, V. A., Guskova, O. A. The effect of alkyl substitutes on the characteristics of charge transfer in stacks of D-π-A-π-D molecules. Rev. Adv. Chem. 2022, 12, 214–221. [CrossRef]
- Raychev, D., Méndez López, R. D., Kiriy, A., Seifert, G., Sommer, J.-U., Guskova, O. Copolymers of diketopyrrolopyrrole and benzothiadiazole: Design and function from simulations with experimental support. Macromolecules 2019, 52, 904–914. [CrossRef]
- Makarova, M. V., Semenov, S. G., Guskova, O. A. Computational study of structure, electronic, and microscopic charge transport properties of small conjugated diketopyrrolopyrrole-thiophene molecules. Int. J. Quant. Chem. 2016, 116, 1459–1466. [CrossRef]
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision A.01. 2009, Gaussian, Inc., Wallingford CT.
- BIOVIA, Dassault Systèmes, Materials Studio 09. 2019, San Diego: Dassault Systèmes.
- Tian, Z., Wen, J., Ma, J. Reactive molecular dynamics simulations of switching processes of azobenzene-based monolayer on surface. J. Chem. Phys. 2013, 139, 014706. [CrossRef]
- Riddick, J. A., Bunger, W. B. Organic Solvents: Physical Properties and Methods of Purification, 3rd ed.; Wiley-Interscience: New York,, 1986; pp. 66–69.
- Savchenko, V., Lomadze, N., Santer, S., Guskova, O. Spiropyran/Merocyanine Amphiphile in Various Solvents: A Joint Experimental–Theoretical Approach to Photophysical Properties and Self-Assembly. Int. J. Mol. Sci. 2022, 23, 11535.
| Reorganization energy | trans-BT-Azo | cis-BT-Azo | trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| [eV] | 1.030 | 0.919 | 0.584 | 0.499 |
| [eV] | 0.309 | 0.214 | 0.366 | 0.826 |
| Contributions [kcal mol] | trans-BT-Azo | cis-BT-Azo | trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| (a) CHCl | ||||
| ideal term | -3.19 | -7.54 | -7.13 | -7.46 |
| van der Waals term | -15.96 | -15.49 | -17.73 | -16.53 |
| electrostatic term | 1.72 | 5.74 | 5.67 | 5.72 |
| -17.34±0.69 | -17.30±0.51 | -19.19±0.72 | -18.26±0.94 | |
| (b) HO | ||||
| ideal term | -3.62 | -3.94 | -7.48 | -7.80 |
| van der Waals term | 6.32 | 6.18 | 5.87 | 4.71 |
| electrostatic term | -1.53 | -1.54 | 1.87 | 1.70 |
| 1.16±0.05 | 0.71±0.04 | 0.26±0.05 | -1.39±0.09 |
| Property | trans-BT-Azo | cis-BT-Azo | trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| [eV] | 0.139±0.115 | 0.205±0.128 | 0.179±0.112 | 0.162±0.167 |
| [eV] | 0.189±0.119 | 0.189±0.153 | 0.168±0.122 | 0.273±0.192 |
| [nm] | 0.75±0.22 | 0.69±0.16 | 0.67±0.13 | 0.66±0.12 |
| k10 [s] | 0.014 | 0.096 | 2.392 | 4.849 |
| k10 [s] | 53.328 | 161.569 | 22.228 | 0.443 |
| [cm V s] | 1.54910 | 8.89310 | 0.209 | 0.411 |
| [cm V s] | 5.840 | 1.497 | 1.942 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





